What Is the Importance of Enthalpy?
- Measuring the change in enthalpy allows us to determine whether a reaction was endothermic (absorbed heat, positive change in enthalpy) or exothermic (released heat, a negative change in enthalpy.)
- It is used to calculate the heat of reaction of a chemical process.
- Change in enthalpy is used to measure heat flow in calorimetry.
How do you calculate enthalpy given the temperature?
May 21, 2020 · Why do we measure enthalpy? Enthalpy is important because it tells us how much heat (energy) is in a system. Heat is important because we can extract useful work from it. In terms of a chemical reaction, an enthalpy change tells us how much enthalpy was lost or gained, enthalpy meaning the heat energy of the system. Click to see full answer.
What is the relationship between enthalpy and entropy?
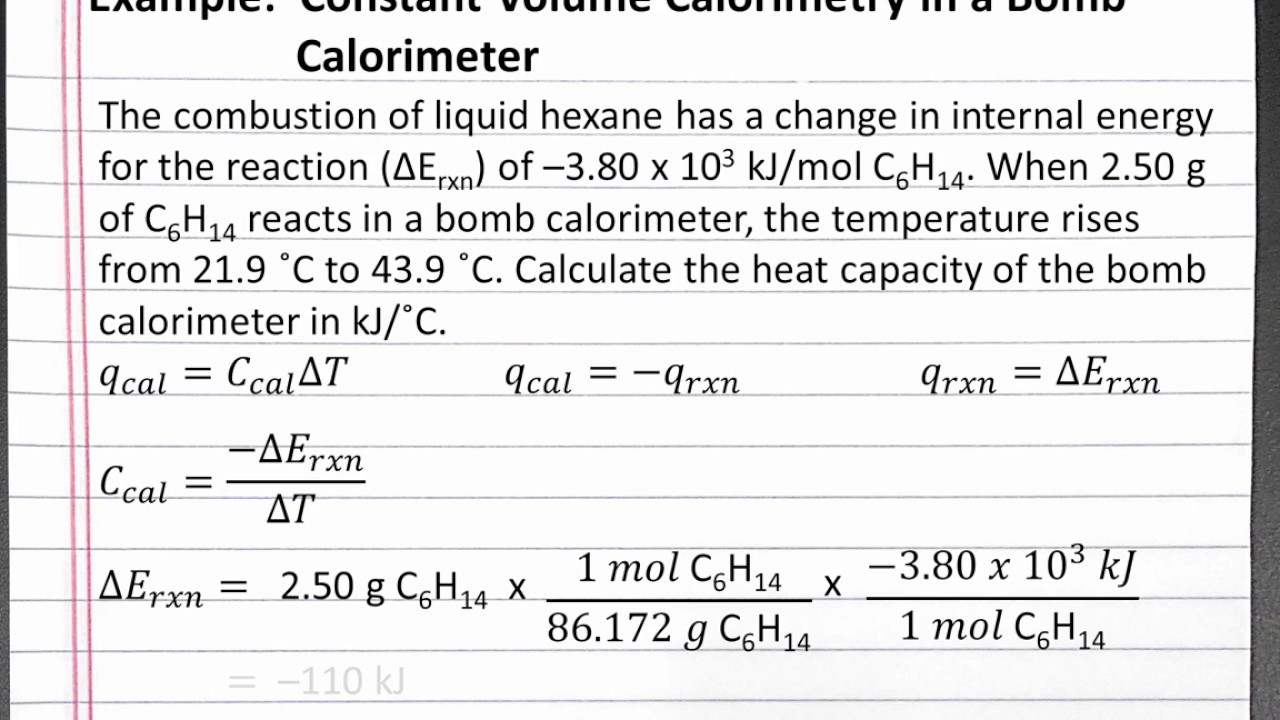
Generally, measurement of enthalpy and internal energy is done by an experimental technique known as calorimetry. Calorimetry techniques are based on thermometric methods carried out in a vessel called calorimeter which is immersed in a known volume of liquid. The heat evolved in the process is generally calculated with the help of known heat capacities of the liquid and the …
How do you calculate the enthalpy of reaction?
We define everything in physics because it's useful. In this case it's useful when a fluid flows in a steady state system and you want to look at energy flows. If a fluid flows through a box, and has a change in specific enthalpy Δ h, with a flow rate of m ˙, …
What is the difference between enthalpy and energy?
Jan 17, 2021 · We normally measure the enthalpy of chemical reaction in order to determine whether the reaction is either endothermic (those that absorb heat) or rather exothermic (release heat). Measuring of enthalpy also the other hand enables one to predict the temperature change during a specific reaction as well as determining how much various reactants are …
What is enthalpy a measure of?
Enthalpy is an energy-like property or state function—it has the dimensions of energy (and is thus measured in units of joules or ergs), and its value is determined entirely by the temperature, pressure, and composition of the system and not by its history.
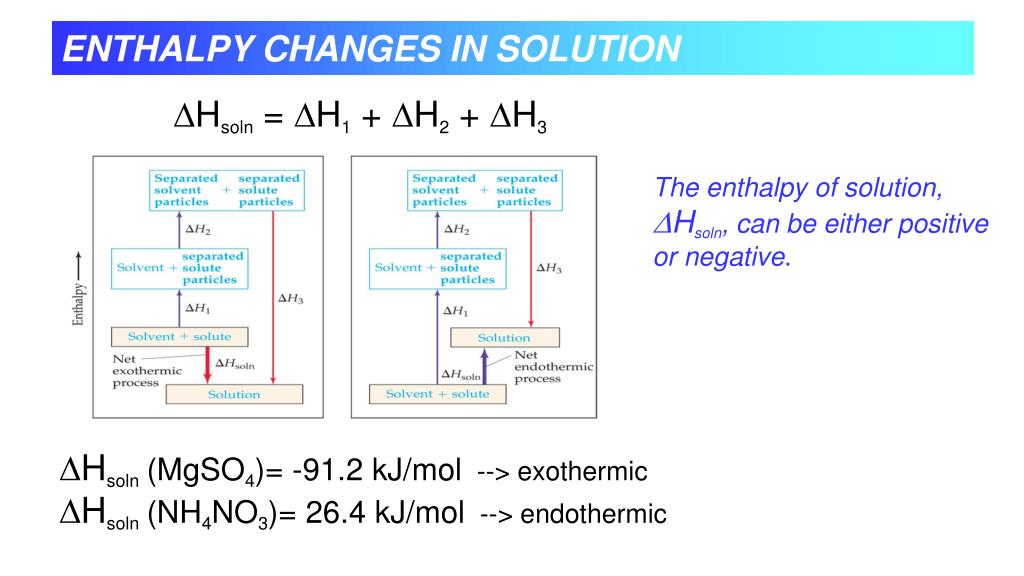
What does enthalpy of solution tell you?
The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at constant pressure). This enthalpy of solution (ΔHsolution) can either be positive (endothermic) or negative (exothermic).Feb 16, 2021
Where is enthalpy used in real life?
Change in enthalpy can apply to refrigerators and hand warmers. In a fridge, refrigerants such as Freon are evaporated. The enthalpy of vaporization (liquid to gas energy change) is equivalent to the coldness of your food. Some people use chemical heat packs (hand warmers) outside.Nov 3, 2020
What factors might affect the value of the enthalpy of dissolution?
The size of the hydration enthalpy is governed by the amount of attraction between the ions and the water molecules.The attractions are stronger the smaller the ion. For example, hydration enthalpies fall as you go down a group in the Periodic Table. ... The attractions are stronger the more highly charged the ion.Aug 21, 2020
Why is enthalpy of solution for some substances negative while for others it is?
This enthalpy of solution (ΔHsolution) can either be positive or negative based on the type of reaction. It is positive when the reaction is endothermic and negative when the reaction is exothermic.
What is enthalpy in refrigeration?
In dealing with liquid and gas pressures in the refrigeration cycle process, never do anything that might result in hydrostatic pressure. Heat content is also known as enthalpy. It is a measure of how much heat a gas or liquid can hold and how much heat is needed to change the temperature.
What is difference between exothermic and endothermic reactions?
In simple terms, the endothermic reactions absorb energy from the surrounding that is in the form of heat. On the other hand, an exothermic reaction releases energy into the surrounding of the system.
What factors influence the rate of reaction?
The factors that affect reaction rates are:surface area of a solid reactant.concentration or pressure of a reactant.temperature.nature of the reactants.presence/absence of a catalyst.
Want to see this answer and more?
Experts are waiting 24/7 to provide step-by-step solutions in as fast as 30 minutes!*
General Chemistry
Learn more about this topic with our new Knowledge Booster section down below.
Measurement of the enthalpy change
The change in enthalpy can be measured by the difference between final enthalpy (enthalpy of products) and initial enthalpy (enthalpy of reactants).
Specific enthalpy
Specific enthalpy is defined as enthalpy per unit mass of a substance.
Effect of temperature on enthalpy
As temperature increases, the K.E of particles in a system increases. This gives rise to internal energy and eventually, enthalpy (H) also increases.
Enthalpy of phase transitions
It is the enthalpy change when 1 mole of gaseous atoms are formed from constituent elements. It is denoted by ∆H atm.
