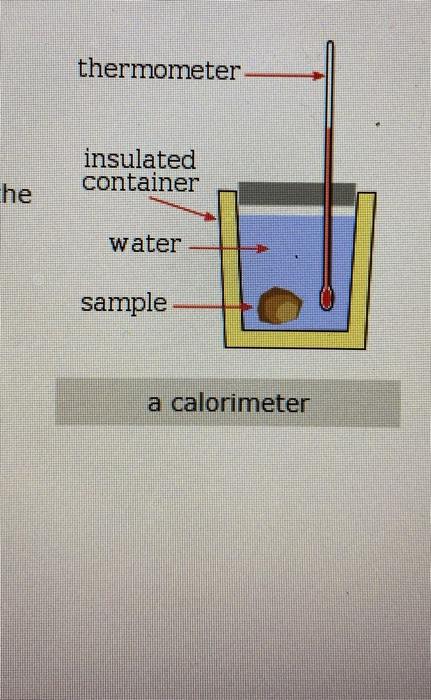
Why is calorimeter covered with wooden lid?
Answer: Heat loss due to conduction is prevented by placing the calorimeter box in a well-lagged vessel using wool or cork material. Heat loss due to convection is prevented by placing a lid on the box.
Is calorimeter open or closed?
Answer: An open system can exchange both matter and energy with the surroundings. ∆ H is measured when an open system is used as a calorimeter. A closed system has a fixed amount of matter, but it can exchange energy with the surroundings.
What happens if the calorimeter is not covered properly?
1) If the calorimeter is not properly insulated then the heat from the calorimeter can be lost to the surroundings.
Why is it important to stir the water in the calorimeter?
Well, so as to prevent hot-spots, and burning on the bottom of the pan. When you do a calorimetric experiment, you have a given mass of water, and you want the temperature rise of this mass to be uniform. So you stir it.
Why is a closed system better than an open system?
An open system can exchange both energy and matter with its surroundings. The stovetop example would be an open system, because heat and water vapor can be lost to the air. A closed system, on the other hand, can exchange only energy with its surroundings, not matter.
Is calorimeter a closed or isolated system?
A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature.
Why is it important to have enough water in the calorimeter to completely cover the metal sample?
Water has a high specific heat, which means it is difficult to increase the temperature of water. However, water also has the capacity to retain heat, which allows the other substance inside the calorimeter to absorb that heat.
What are some possible sources of error in a calorimetry experiment?
The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. This can be reduced by insulating the sides of the calorimeter and adding a lid.
What are the precautions and conditions for a calorimetric experiment?
PrecautionsDo not stir the liquid in the calorimeter vigorously because vigorous stirring does cause some increase in temperature.The space between the calorimeter and the wooden box should be filled with cotton to avoid heat loss.
Why do we need to stir the water bath?
Uniformity and stability Temperature-bath with stirring of the water will improve the uniformity of the temperature.
What happens when we do not stir the mixture?
1 Answer. Explanation: If temp of mixture (T) is not constant then according to calculation we find wrong value of specific heat. If we stir continuously then temperature of whole mixture becomes same.
Why do you need to stir the bath continuously?
Shaking water baths This type of water bath has extra control for shaking, which moves liquids around. This shaking feature can be turned on or off. In microbiological practices, constant shaking allows liquid-grown cell cultures grown to constantly mix with the air.
How do you know that a sealed calorimeter is a closed system?
The Bomb Calorimeter becomes a closed system if you don't count the surrounding environment since there is energy flow from the rxn to the water.
What type of system is a calorimeter?
A bomb calorimeter is a closed system because it allows heat to be exchanged. While this system is insulated, an "insulated system" is not one of the main three types of systems: closed, open, and isolated. An isolated system would not allow for any transfer of matter or energy.
How does a calorimeter work?
A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature.
What are the types of calorimeter?
Types of CalorimeterAdiabatic Calorimeters.Reaction Calorimeters.Bomb Calorimeters (Constant Volume Calorimeters)Constant Pressure Calorimeters.Differential Scanning Calorimeter.
How to get more reliable results from calorimetry?
More reliable results can be obtained by repeating the experiment many times . The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. This can be reduced by insulating the sides of the calorimeter and adding a lid.
Why is the spirit burner weighed before and after the experiment?
The spirit burner containing the fuel is usually weighed before and after the experiment so that the mass of the fuel burned can be found.
What is the measurement of heat energy?
Calorimetry - measuring energy changes from combustion. Energy can be released in chemical reactions as light, sound or electrical energy. But it is most often released as heat energy. Measuring heat transfers is called calorimetry. The diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel:
What is the measurement of cold water?
Cold water is measured into a copper calorimeter (a small metal can).
How to compare fuels?
When comparing different fuels, it is important to carry out a fair test. Several variables should be kept constant. They include: 1 the volume of water used 2 the starting temperature of the water 3 the temperature increase 4 the distance of the flame from the calorimeter
