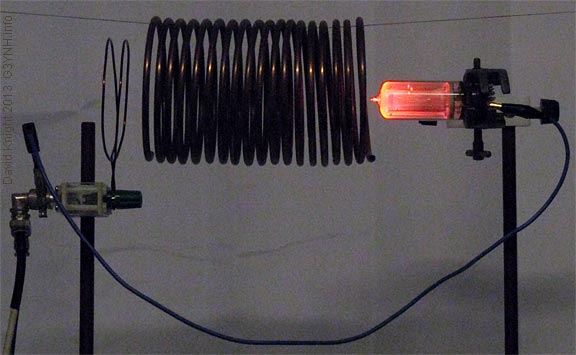
When the wavelength of the radiation is between 20 Hz to 20 kHz, it is visible. Any gas discharge tube has a fixed color because one particular spectra has the same frequency or wavelength which depends on the kind of gas used. The electrons get transferred through the gas from the cathode to the anode.
Which gases are excited in a veritical tube?
Veritical tubes filled with the following gases are excited. What colors do they glow? 1. Hydrodgen 2. Helium 3. Neon 4. Argon 5. Krypton 6. Xenon
Why is the light different in each tube of light?
The light is differently colored in each tube because of the different wavelengths of light that must be released in each instance. Different elements emit different wavelengths of light to return to their respective ground states, so the tubes' colors are varied.
What is a gas tubes?
Nihal Kularatna, ... Sisira James, in Design of Transient Protection Systems, 2019 GDTs (or simply gas tubes) are devices that employ an internal inert gas that ionizes and conducts during a transient event. Because the internal gas requires time to ionize, gas tubes can take several microseconds to turn on or ‘fire’.
Which gases glow when the Tesla coil connects?
Oxygen 9. Water Vapor The tubes glow their respective colors when the Tesla coil connects with them. The video shows the gases in the numerical order presented in the tables above. The Tesla coil is an energy source. This energy excites the electrons in the gases to higher energy states.
What happens when the electric field in a discharge tube increases from zero?
How are phosphors converted to visible light?
What is plasma diagnostics?
What are the background emission spectrums of helium and argon?
Why do we use doping gases?
What gases are used to determine the group of elements?
How many volts does a gas tube arrestor overshoot?
See 4 more
About this website

Why does a gas discharge tube eg a neon light have a certain color?
The ultraviolet light emitted by the mercury discharge inside a tube is absorbed by the coating, which subsequently emits light of a different color (and with a lower photon energy). Depending on the exact material of the coating, a whole range of colors can be obtained.
Which gas is used in colour discharge tube?
neonColoured lighting in discharge tubes are usually filled with neon. Mixing with other gases results in different coloured lights.
How does a gas discharge tube work?
Gas discharge tubes dissipate voltage transients through a contained plasma gas. They have high insulation resistance plus low capacitance and leakage to ensure minimal effect on normal operation of equipment.
Which type of spectrum would you expect to see from a gas discharge tube?
Discharge tubes containing samples of various elemental gases are placed in a high-voltage power supply. Students observe the emitted light through diffraction gratings. They see discrete line spectra corresponding to the energy level transitions of the electrons of the atoms of the element.
Why do gases have different colours?
The light is differently colored in each tube because of the different wavelengths of light that must be released in each instance. Different elements emit different wavelengths of light to return to their respective ground states, so the tubes' colors are varied.
What is the purpose of discharge tube?
A specialized type of gas-filled tube called a Gas Discharge Tube (GDT) is fabricated for use as surge protectors, to limit voltage surges in electrical and electronic circuits. Due to their very low capacitance, gas discharge tubes are commonly used on high frequency lines, like in telecommunications equipment.
How do I choose a gas discharge tube?
Types of Gas Discharge Tube Performance CharacteristicsCapacitance range is the range of ability a gas discharge tube has to hold an electrical charge. ... Sparkover voltage is the main characteristic defining a GDT. ... Holdover voltage is the voltage for which the gas tube was designed to operate optimally and safely.More items...
Do gas discharge tubes have polarity?
You may assume the dot in the schematic symbol indicates a polarity. But most gas discharge tubes are polarity insensitive, and the dot signifies that the device is gas filled (usually at some pressure lower than 1 atmosphere).
What is a gas discharge tube made of?
A discharge tube is an object that contains a certain arrangement of electrodes within a gas chamber. Usually, these objects are designed within glass shielding; however, some use ceramic or glass-lined metal.
What type of gaseous discharge lamp emits a blue green color?
It gives a characteristic blue-green light due to emission at select wavelengths. Wavelengths of spectral emission by different gases due to electrical discharges are shown in Table 1.4....1.8. 3 Gas-discharge lamps.Gas/vapourWavelengths of emission (nm)Helium412, 439, 447, 471, 492, 588, 688, 706 and 728.4 more rows
Which gas produces a bluish white Coloured light in a discharge tube?
ColorGasColorWater vaporSimilar to hydrogen, dimmerCarbon dioxideBlue-white to pink, in lower currents brighter than xenonMercury vaporLight blue, intense ultravioletSodium vapor (low pressure)Bright orange-yellow8 more rows
How does an emission spectrum of a gas in a discharge tube differ from a white light spectrum?
The emission spectrum of a gas in a discharge tube differs from the white light spectrum because the emission spectrum arises when an atom absorbs energy and that energy is re-emitted as light. The light that is emitted consists of distinctive wavelengths for every element.
Which gas is pink in colour?
hydrogenFrom green to pink hydrogen, we reveal the rainbow of hydrogen colours and the different types of technology used to produce each.
Why noble gases are used in discharge tube?
Solution : Noble gases are used in discharge tubes to give different colours. Reddish orange glow is due to Ne.
What colour is helium gas?
helium (He), chemical element, inert gas of Group 18 (noble gases) of the periodic table. The second lightest element (only hydrogen is lighter), helium is a colourless, odourless, and tasteless gas that becomes liquid at −268.9 °C (−452 °F).
What colour is nitrogen?
blueTypical assignmentshydrogen (H)whitenitrogen (N)blueoxygen (O)redfluorine (F), chlorine (Cl)greenbromine (Br)dark red11 more rows
How does a gas discharge tube work? - Quora
Answer (1 of 3): A gas-filled tube, also known as a discharge tube, is an arrangement of electrodes in a gas within an insulating, temperature-resistant envelope. Gas-filled tubes exploit phenomena related to electric discharge in gases, and operate by ionizing the gas with an applied voltage suf...
ac - How to use Gas Discharge Tubes, Transils and other goodies ...
I would like to make a good surge protector for a device that will have a hardwired mains connection. The protected electronics will consist of a ~10W SMPS and a low-power AC load (<100W) controlled with a triac.
First Principles of a Gas Discharge Tube (GDT) Primary Protector
ii Page Figure 1 GDT switching characteristic 4 Figure 2 Ramp impulse voltage vs DCBD voltage options 5 Figure 3 2026 series surge impulse voltage during 1 kV, 100 A 10/1000 µs 6 Figure 4 DCBD voltage vs 1000 V/µs ramp impulse voltage 7 Figure 5 Variation of 2026-35 with 100 A 10/1000 µs surge 9 Figure 6 2026-35 GDT switching characteristic with 1 kV, 100 A 10/1000 µs surge 10
Gas Discharge Tubes (GDT) | Circuit Protections - Unictron
Gas Discharge Tubes Abbreviated as GDT . Gas discharge tube is the most widely used switching device in lightning protection equipment. It is connected in series and parallel in the circuit and can be used for lightning protection of AC and DC power supplies and various signal circuits.
What happens when the electric field in a discharge tube increases from zero?
As the electric field in a discharge tube increases from zero, a small dark current ( Townsend discharge) is drawn, but at some point there is a sudden transition to one of the several forms of self-sustaining discharge. Figure 7 shows the critical breakdown voltage as a function of the pressure–distance product, where the distance is measured between the electrodes.
How are phosphors converted to visible light?
Ultraviolet radiation reaching the walls of the discharge tube is absorbed and converted to visible light by coatings of luminescent phosphor powders applied to them . Such phosphors are composed of inorganic crystalline materials, optically transparent in the pure state but synthesized to incorporate specific luminescent centers to absorb the desired wavelengths of UV light and emit visible rays. Such luminescent centers are typically strongly coupled to ligand fields of the solid; therefore, excitation by a UV photon creates excited state complexes in high vibrational energy states. These interact with lattice phonons to relax states of the excited manifold to lower vibrational energy before radiating. As a result of the loss of energy by phonon relaxation, the energy of the photon emitted on re-radiation is substantially less than that of the absorbed one. Phosphor development for application to fluorescent lamps has been an active area of research since 1934, when the first experimental fluorescence lamps were made. The discovery in 1942 of calcium halophosphate phosphors, with emission over a broad band of visible wavelengths, was a major breakthrough in the industry. These phosphors are still used in fluorescent lamps, since they are relatively inexpensive and provide CRI in the range 51–76. The discovery of rare-earth phosphors in the mid-70 s, with narrow emission bands at 610 nm in the red, 545 nm in the green, and 450 nm in the blue, led to the development of three band phosphors with CRI values between 80 and 85. More recently, multiband phosphors have been developed with CRI values of 90 and above, but with lower efficacies compared to three-band phosphors.
What is plasma diagnostics?
Plasma diagnostics is the observation of physical processes that allows one to infer parameters that characterize a plasma . It had its inception in the late nineteenth century with the observation of colored glows from gas-filled discharge tubes. These plasmas were low in temperature and weakly ionized.
What are the background emission spectrums of helium and argon?
The background emission spectrum due to the plasma is low for both helium and argon and the spectra of both these gases are well characterized so that specific spectral lines may be avoided. If a particularly low background is needed, wavelength modulation may be used. Other spectral interferences are due to impurities from the support gases and the atmosphere such as carbon dioxide, nitrogen gas, and water vapor, or impurities from the sample introduction system and discharge tube. Memory effects are also a problem due to analyte atoms collecting in etched areas of the discharge tubes.
Why do we use doping gases?
The use of doping gases to overcome matrix effects, especially when the MIP is being used as a gas chromatographic detector, is widespread. The gases act as scavengers and are added to prevent carbon from depositing on the wall of the discharge tube, because if this occurs severely distorted chromatographic peaks may be obtained. Different gases are used for the determination of different groups of elements. For the simultaneous determination of carbon, hydrogen, chlorine, and bromine, oxygen is the chosen gas; but if elements that form refractory compounds are present, hydrogen would be needed.
What gases are used to determine the group of elements?
Different gases are used for the determination of different groups of elements. For the simultaneous determination of carbon, hydrogen, chlorine, and bromine, oxygen is the chosen gas; but if elements that form refractory compounds are present, hydrogen would be needed. View chapter Purchase book. Read full chapter.
How many volts does a gas tube arrestor overshoot?
A circuit protected by a gas tube arrestor will typically see overshoot voltages ranging from a few hundred volts to several thousand volts.
What happens when the electric field in a discharge tube increases from zero?
As the electric field in a discharge tube increases from zero, a small dark current ( Townsend discharge) is drawn, but at some point there is a sudden transition to one of the several forms of self-sustaining discharge. Figure 7 shows the critical breakdown voltage as a function of the pressure–distance product, where the distance is measured between the electrodes.
How are phosphors converted to visible light?
Ultraviolet radiation reaching the walls of the discharge tube is absorbed and converted to visible light by coatings of luminescent phosphor powders applied to them . Such phosphors are composed of inorganic crystalline materials, optically transparent in the pure state but synthesized to incorporate specific luminescent centers to absorb the desired wavelengths of UV light and emit visible rays. Such luminescent centers are typically strongly coupled to ligand fields of the solid; therefore, excitation by a UV photon creates excited state complexes in high vibrational energy states. These interact with lattice phonons to relax states of the excited manifold to lower vibrational energy before radiating. As a result of the loss of energy by phonon relaxation, the energy of the photon emitted on re-radiation is substantially less than that of the absorbed one. Phosphor development for application to fluorescent lamps has been an active area of research since 1934, when the first experimental fluorescence lamps were made. The discovery in 1942 of calcium halophosphate phosphors, with emission over a broad band of visible wavelengths, was a major breakthrough in the industry. These phosphors are still used in fluorescent lamps, since they are relatively inexpensive and provide CRI in the range 51–76. The discovery of rare-earth phosphors in the mid-70 s, with narrow emission bands at 610 nm in the red, 545 nm in the green, and 450 nm in the blue, led to the development of three band phosphors with CRI values between 80 and 85. More recently, multiband phosphors have been developed with CRI values of 90 and above, but with lower efficacies compared to three-band phosphors.
What is plasma diagnostics?
Plasma diagnostics is the observation of physical processes that allows one to infer parameters that characterize a plasma . It had its inception in the late nineteenth century with the observation of colored glows from gas-filled discharge tubes. These plasmas were low in temperature and weakly ionized.
What are the background emission spectrums of helium and argon?
The background emission spectrum due to the plasma is low for both helium and argon and the spectra of both these gases are well characterized so that specific spectral lines may be avoided. If a particularly low background is needed, wavelength modulation may be used. Other spectral interferences are due to impurities from the support gases and the atmosphere such as carbon dioxide, nitrogen gas, and water vapor, or impurities from the sample introduction system and discharge tube. Memory effects are also a problem due to analyte atoms collecting in etched areas of the discharge tubes.
Why do we use doping gases?
The use of doping gases to overcome matrix effects, especially when the MIP is being used as a gas chromatographic detector, is widespread. The gases act as scavengers and are added to prevent carbon from depositing on the wall of the discharge tube, because if this occurs severely distorted chromatographic peaks may be obtained. Different gases are used for the determination of different groups of elements. For the simultaneous determination of carbon, hydrogen, chlorine, and bromine, oxygen is the chosen gas; but if elements that form refractory compounds are present, hydrogen would be needed.
What gases are used to determine the group of elements?
Different gases are used for the determination of different groups of elements. For the simultaneous determination of carbon, hydrogen, chlorine, and bromine, oxygen is the chosen gas; but if elements that form refractory compounds are present, hydrogen would be needed. View chapter Purchase book. Read full chapter.
How many volts does a gas tube arrestor overshoot?
A circuit protected by a gas tube arrestor will typically see overshoot voltages ranging from a few hundred volts to several thousand volts.
