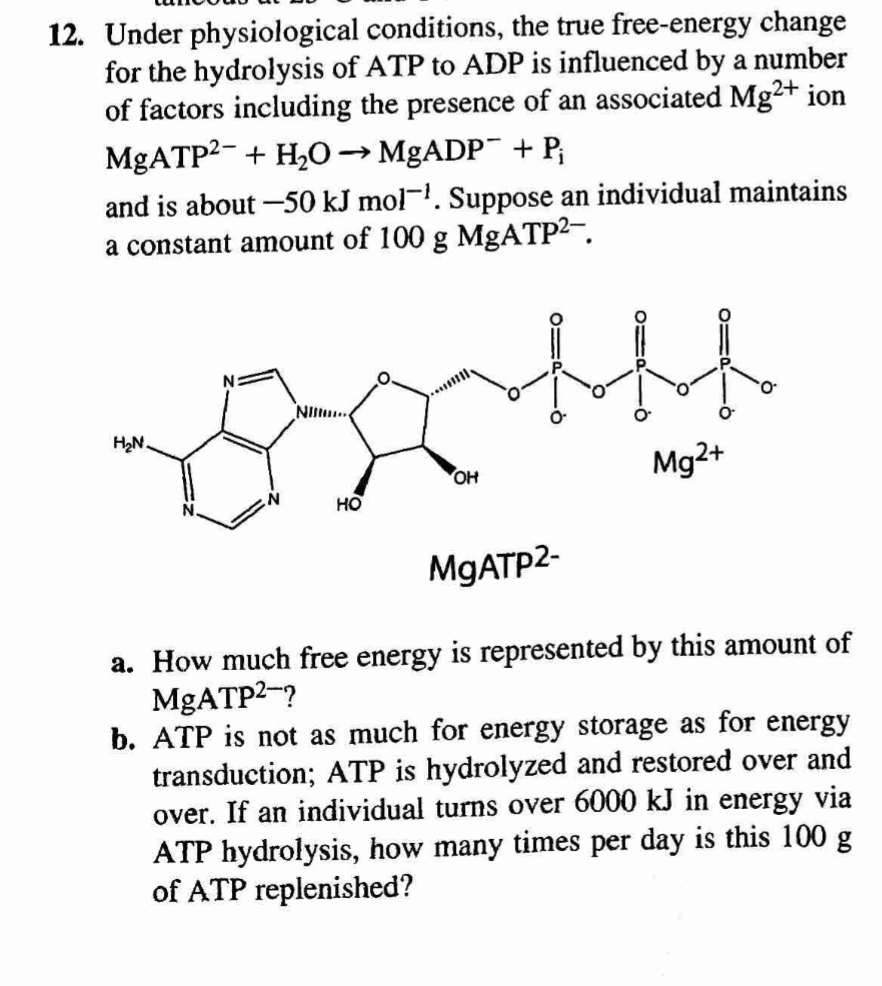
Adenosine triphosphate
Adenosine triphosphate is a complex organic chemical that provides energy to drive many processes in living cells, e.g. muscle contraction, nerve impulse propagation, and chemical synthesis. Found in all forms of life, ATP is often referred to as the "molecular unit of currency" of i…
What happens when ATP is turned into ADP?
What happens when ATP becomes ADP? If a cell needs to spend energy to accomplish a task, the ATP molecule splits off one of its three phosphates, becoming ADP (Adenosine di-phosphate) + phosphate. The energy holding that phosphate molecule is now released and available to do work for the cell.
What occurs when ATP is converted to ADP?
What is the most efficient way to produce ATP?
- Aerobic respiration is far more energy-efficient than anaerobic respiration.
- Aerobic processes produce up to 38 ATP per glucose.
- Anaerobic processes yield only 2 ATP per glucose.
What releases energy when ATP changes to ADP?
Why is ATP hydrolysis an exergonic reaction?
- The entropy, which is the level of disorder, of ADP is greater than that of ATP. ...
- Electrostatic repulsion of the four negative charges on the oxygens of the ATP molecule. ...
- Resonance stabilization of ADP and of P i is greater than that of ATP. ...
- There is a greater degree of solvation of P i, H +, and ADP, relative to ATP. ...
Does ATP have a higher energy than ADP?
Yes. ATP, using its three phosphate groups, has more stored chemical energy than does ADP. Why is ADP entropy higher than ATP? The entropy, the degree of disorder, of ADP is more than those of ATP. Therefore, because of thermodynamics, the response spontaneously occurs since it really wants to attend a greater entropy level.
Why is energy released when ATP is converted to ADP?
Energy is released because the products (ADP and phosphate ion) have less energy than the reactants [ATP and water (H2O)]. If the hydrolysis of ATP releases energy, its synthesis (from ADP) requires energy.
Why does ATP release energy easily?
The reason there is energy released in the process is because the products formed (ADP and hydrogenphosphate/phosphate) have stronger covalent bonds (plus intermolecular forces with the surrounding solution and dissolved ions) than the starting materials. This is the case for any exothermic process.
How does ATP ADP store and release energy?
ATP or Adenosine triphosphate acts as the energy currency of the cell. It stores the energy released in the oxidation of glucose during cellular respiration. Energy is stored in the form of high energy phosphate bonds, which is released when it is broken. ATP is broken into ADP and Pi and energy is released.
Why does the conversion of ATP to ADP keep us warm?
Explanation. The conversion of ATP to ADP help keep us warm because, during the separation of an inorganic phosphate molecule (Pi), most of the energy is released as heat.
Why is ADP more stable than ATP?
Resonance stabilization of ADP and of Pi is greater than that of ATP. The oxygen molecules of the ADP are sharing electrons. Those electrons are constantly being passed back and forth between the oxygens, creating an effect called resonance. This stables the ADP.
How does ATP release energy that is stored within the molecules?
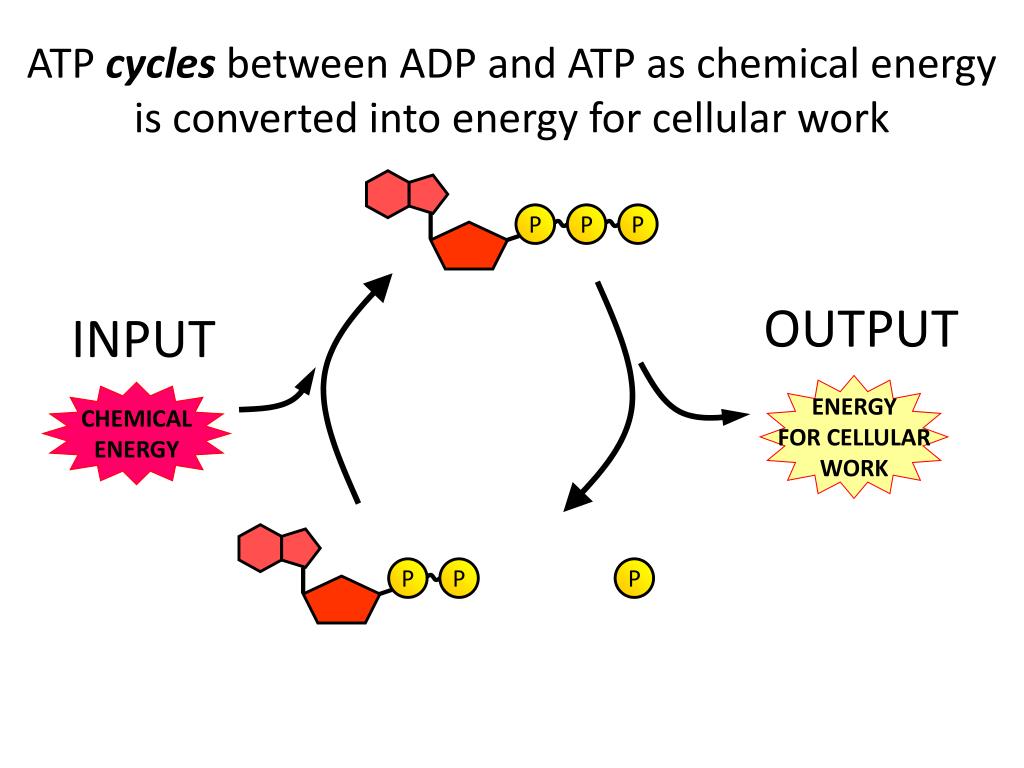
When the cell needs energy to do work, ATP loses its 3rd phosphate group, releasing energy stored in the bond that the cell can use to do work. Now its back to being ADP and is ready to store the energy from respiration by bonding with a 3rd phosphate group. ADP and ATP constantly convert back and forth in this manner.
Why does the phosphate end of ATP store energy?
Explain why the phosphate end of ATP stores potential energy? Each of the phosphate groups is negatively charged. These negatives repel each other and so they would have less energy if they were further apart.
How does ATP release energy that can be used by living cells?
ATP is able to power cellular processes by transferring a phosphate group to another molecule (a process called phosphorylation). This transfer is carried out by special enzymes that couple the release of energy from ATP to cellular activities that require energy.
Why is ATP hydrolysis so favorable?
1) Getting rid of one phosphate group is energetically favourable because there is a large electrostatic repulsion between phosphate groups in ATP (see picture above) because they are very close together, and all have various negative charges on the oxygens (like charges repel, opposite charges attract).
How does ATP release energy that can be used by living cells quizlet?
ATP can easily release and store energy by breaking and reforming the bonds between its phosphate groups. This characteristic of ATP makes it exceptionally useful as a basic energy source for all cells.
How does ATP release energy that's stored within the molecule?
When the cell needs energy to do work, ATP loses its 3rd phosphate group, releasing energy stored in the bond that the cell can use to do work. Now its back to being ADP and is ready to store the energy from respiration by bonding with a 3rd phosphate group. ADP and ATP constantly convert back and forth in this manner.
Why the hydrolysis of ATP is a favorable reaction?
In the first reaction, a phosphate group is transferred from ATP to glucose, forming a phosphorylated glucose intermediate (glucose-P). This is an energetically favorable (energy-releasing) reaction because ATP is so unstable, i.e., really "wants" to lose its phosphate group.
Why is ATP and ADP maintained out of equilibrium in cells?
And second because ATP and ADP are maintained out of equilibrium in cells. The action of utilizing an ATP brings the system closer to equilibrium, which releases energy. There is a required energy input to break the phosphate to phosphate bond. This is the activation energy.
What is the bond formed by hydrolysis of ATP?
Hydrolysis of ATP (or any other compound) is not only breaks the O-P bond but also forms new bonds. ATP + H 2 O --> ADP + Pi. A new bond is formed between the O of water (the nucleophile) and the P of the second phosphoryl group (the electrophile). Hydrolysis of ATP is both exothermic (delta H is negative) and exergonic (delta G is negative).
What is the FoF1-ATP synthetase?
In the case of ATP synthesis, it should be borne in mind that FoF1-ATP synthetase is a nanomachine that receives energy thanks to proton currents which it mechanically transfers to ADP Pi, exerting a sort of constraint effect on ADP Pi leading to the overlap of molecular orbitals.
What energy is needed to break a phosphate to phosphate bond?
There is a required energy input to break the phosphate to phosphate bond. This is the activation energy . However, the energy released upon hydrolysis and moving the system towards equilibrium are of greater magnitude than the activation energy input.
What happens when energy is added to a reaction?
When energy is added to a reaction or removed from a reaction where a covalent bond is made or broken, it is the electrons that are gaining and loosing energy and moving to "excited" orbitals to facilitate the formation or dissolution of the covalent bond.
Is ATP an exothermic reaction?
Reactions can be either exergonic (exothermic) or endergonic (endothermic) depending on the free energy difference (delta G) between substrates and products. In the case of the hydrolysis of ATP to ADP + inorganic phosphate under typical aqueous conditions, the reaction is exothermic, releasing energy.
Does breaking covalent bonds release energy?
The textbook explained that forming covalent bonds releases energy. On the other hand, breaking covalents requires the released energy to be absorbed. But in the case of forming ATP, energy is required to form the bond between an extra phosphate with ADP to form ATP. Furthermore, the breakdown of ATP to ADP releases energy.