Why does carbon dioxide diffuse faster than oxygen in the lungs?
In the lungs, whilst oxygen is smaller than carbon dioxide, the difference in solubility means that carbon dioxide diffuses roughly 20 times faster than oxygen. Normally this is compensated by the large difference in partial pressures of oxygen, however, this means that in disease, oxygen exchange is often compromised before that of carbon dioxide.
What is the rate of diffusion of CO2?
The rate of diffusion is inversely proportional to the square root of molecular masses. It means CO diffuses 1.25 times the rate of diffusion of CO2. As CO has less molecular weight, it diffuses faster. What diffuses faster CO2 or CO?
Why does hydrogen diffuse faster than oxygen?
According to Graham's law of diffusion, the rate of diffusion or movement of gas is inversely proportional to the square root of it's molecular weight. Among H2 , O2 , CO, and N2 , the gas with least mass is hydrogen. So hydrogen diffuses faster whereas oxygen takes long time to diffuse.
Which gas diffuses the fastest?
When gases are diffusing through liquids, for example across the alveolar membrane and into capillary blood, the solubility of the gases is important. The more soluble a gas is, the faster it will diffuse. In this case carbon dioxide diffuses much faster than oxygen, as it is much more soluble. Which is the gas that diffuses the fastest?
Why does CO2 diffuse faster?
Carbon dioxide is inherently more soluble than oxygen, and thus diffuses much faster than oxygen into liquid.
Why is CO2 more soluble than O2 in blood?
The molecular arrangement of carbon dioxide makes it more soluble in blood compared to the solubility of oxygen. The solubility of carbon dioxide is 20-25 times higher than oxygen. Thus, higher the solubility higher will be the rate of diffusion.
Does CO2 diffuse easily?
Because the cell membrane is semipermeable, only small, uncharged substances like carbon dioxide and oxygen can easily diffuse across it.
Why would O2 effuse faster than CO2 in the same room?
Explanation: When gases are diffusing through liquids, for example across the alveolar membrane and into capillary blood, the solubility of the gases is important. The more soluble a gas is, the faster it will diffuse. In this case carbon dioxide diffuses much faster than oxygen, as it is much more soluble.
Does CO2 or O2 dissolve more easily?
Carbon dioxide, also called CO2, is found in water as a dissolved gas. It can dissolve in water 200 times more easily than oxygen.
What is difference between CO2 and O2?
The key difference between oxygen and carbon dioxide is that oxygen is a diatomic molecule having two oxygen atoms whereas carbon dioxide is a triatomic molecule having one carbon atom and two oxygen atoms.
Which will diffuse faster according to Graham's law O2 or CO2?
Carbon dioxide (CO2) diffuses faster. The rate of diffusion of a gas is inversely proportional to the square root of its density/molar mass, as per Graham's law of diffusion.
Does CO2 and O2 diffuse?
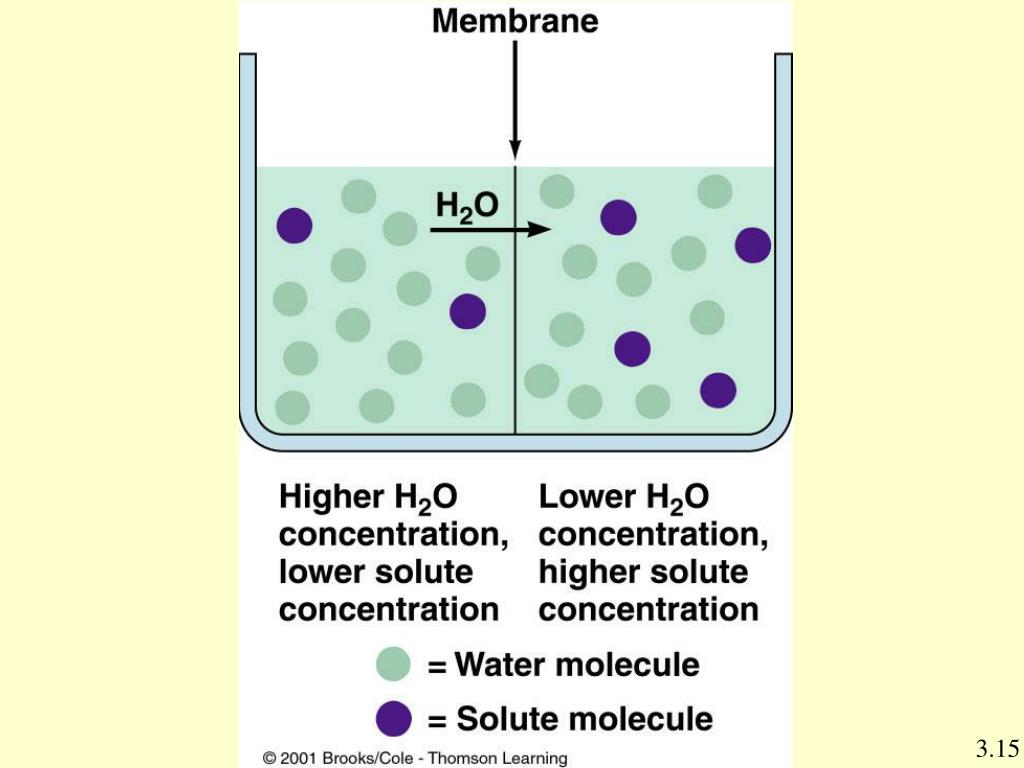
The walls of the alveoli share a membrane with the capillaries. That's how close they are. This lets oxygen and carbon dioxide diffuse, or move freely, between the respiratory system and the bloodstream. Oxygen molecules attach to red blood cells, which travel back to the heart.
Which will diffuse faster CO or CO2?
Answer : CO diffuses faster.
Which gas has the fastest rate of diffusion?
According to Graham's law, the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass.The molar mass of hydrogen gas is 2 amu.Since hydrogen is the lightest gas among these gases, it will have the fastest rate of diffusion.
Which gas will diffuse fastest?
The gas with the lowest molecular weight will effuse the fastest. The lightest, and therefore fastest, gas is helium.
Which of these gases diffuse more quickly than oxygen?
Hence, CH4 will diffuse fast . Was this answer helpful?
Why does CO2 dissolve in blood?
[2] Most of the carbon dioxide diffusing through the capillaries and ultimately into the red blood cells combines with water via a chemical reaction catalyzed by the enzyme carbonic anhydrase catalyzes, forming carbonic acid. Carbonic acid almost immediately dissociates into a bicarbonate anion (HCO3-) and a proton.
Why is it important that CO2 is 20 times as soluble in blood than O2?
Dissolved CO2, like O2, obeys Henry's law, but CO2 is about 20 times more soluble than O2, its solubility being 0.067 ml per dL per mmHg. As a result, dissolved CO2 plays a significant role in its carriage in that about 5-10% of the gas that is evolved into the lung from the blood is in the dissolved form.
Is carbon dioxide more water soluble than oxygen?
Carbon dioxide content in air is only 0.03%, but it is highly soluble in water unlike oxygen and one volume of CO2 dissolves in equal volume of water, the solubility being higher at low temperature (see Table III).
How much radiation does CO2 absorb?
The only radiation from earth’s surface absorbed by CO2 is 15 microns, which is extremely low Planck energy (E=hf) corresponding to a median Planck emission temperature of -80 C.
How fast does hydrogen effuse?
Hydrogen effuses four times as rapidly as oxygen. At a particular pressure and temperature, nitrogen gas effuses at the rate of 79 mL/s.
How many air molecules does CO2 have?
CO2 is 400 ppm or 1/2500 air molecules. For 1/2500 air molemolecules to raise atmospheric temperatures by a mere 1 C it has to become 2500 C itself first.
What causes the thermosphere to cool?
It’s causing thermosphere to cool and shrink, as a consequence, changing drag coefficient of satellites.
Why do H2 molecules diffuse more quickly?
H2 molecules diffuse more quickly. Why? Because they’re lighter. According to kinetic theory, lighter molecules move faster than heavier ones. Open that textbook a little wider and let the knowledge spill out, lol!
Which molecules diffuse faster?
At any given temperature, small, light molecules (such as H2, hydrogen gas) diffuse faster than larger, more massive molecules (such as N 2, nitrogen gas) because they are traveling faster, on the average (see heat; kinetic-molecular theory of gases).
Which molecule diffuses faster, helium or H2?
And the lumps on the end of the stick are each bigger than the helium atom. This is why helium diffuses faster than H2 through solids and also leaks faster through small passages.
Why is the partial pressure of oxygen low in the alveoli compared to the external environment?
This is due to continuous diffusion of oxygen across the alveolar membrane and the diluting effect of carbon dioxide entering the alveoli to leave the body.
Why does blood travel in the pulmonary capillaries during exercise?
During exercise, blood spends up to half the normal time (one second at rest) in the pulmonary capillaries due to the increase in cardiac output moving blood around the body more quickly. However, diffusion of oxygen is complete within half a second of the blood cell arriving in the capillary, which means that exercise is not limited by gas exchange.
What are some examples of fluid in the interstitial space?
Examples include: Fluid in the interstitial space (pulmonary oedema). Thickening of the alveolar membrane (pulmonary fibrosis). Membrane surface area – the larger the surface area, the faster the rate of diffusion. The lungs normally have a very large surface area for gas exchange due to the alveoli.
Why do the lungs have a large surface area?
The lungs normally have a very large surface area for gas exchange due to the alveoli. Diseases such as emphysema lead to the destruction of the alveolar architecture, leading to the formation of large air-filled spaces known as bullae. This reduces the surface area available and slows the rate of gas exchange.
What is the role of gas exchange in emphysema?
This is the primary function of the respiratory system and is essential for ensuring a constant supply of oxygen to tissues, as well as removing carbon dioxide to prevent its accumulation. ...
What is the process of removing carbon dioxide from the bloodstream?
Gas Exchange. Gas exchange is the process by which oxygen and carbon dioxide move between the bloodstream and the lungs. This is the primary function of the respiratory system and is essential for ensuring a constant supply of oxygen to tissues, as well as removing carbon dioxide to prevent its accumulation.
When gases are diffusing through liquids, for example across the alveolar membrane and into capillary blood,?
When gases are diffusing through liquids, for example across the alveolar membrane and into capillary blood, the solubility of the gases is important. The more soluble a gas is, the faster it will diffuse.
Why is O2 dissolving in blood and fluids important?
O2 dissolving into blood and fluids is also important for the uptake of oxygen in the lungs, as the oxygen has to get to the Hb inside the erythrocytes to be carried around. Comment on dysmnemonic's post “Yes and no. We care the most about haemoglobin for...”. Button opens signup modal.
Where does deoxygenated blood flow?
Deoxygenated blood is carried to the alveoli, and it has a high concentration of CO2, when compared with the air that we are breathing in. Therefore, the oxygen flows to its area of low concentration, the dexoygenated blood, and CO2 flows to its area of low concentration, into the alveoli. Comment on Anna's post “Also, concentration gradients ...
Is nitrogen a stable molecule?
Direct link to Roger Gerard's post “Nitrogen (N2) is an extremely stable molecule due ...”. Nitrogen (N2) is an extremely stable molecule due to it's triple bond. Due to it's stability the molecules on Nitrogen that diffuse through the blood do not undergo many, if any, chemical reactions in the blood.
Do medical videos provide advice?
These videos do not provide medical advice and are for informational purposes only. The videos are not intended to be a substitute for professional medical advice, diagnosis or treatment. Always seek the advice of a qualified health provider with any questions you may have regarding a medical condition.
Does hemoglobin help with oxygen carrying capacity?
Yes and no. We care the most about haemoglobin for determining the oxygen carrying capacity of the blood, but the Hb mostly acts as a buffer for dissolved oxygen. When dissolved oxygen is taken out of the blood for cells to use, it's replaced by O2 dissolving back into the blood from Hb.