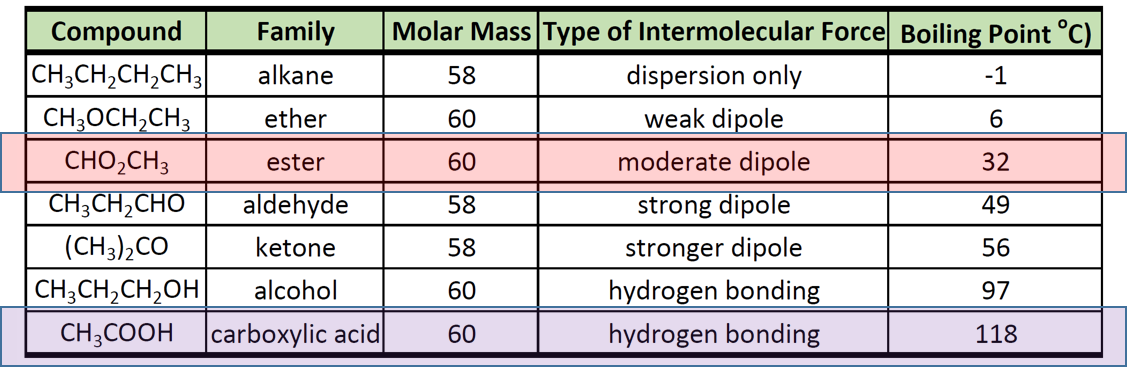
Which has a higher boiling point HI or HCl?
Out of HCl, HBr and HI, the mass of HCl is minimum so it has less magnitude of vander waals forces and hence its boiling point will be least. HCl has lowest boiling point and HF has highest boiling point. Tired of being single? Try this premium dating site today! HI>HBr>HCl>HF because the atomic mass of the halogen decreases in the same order.
Which has a lower melting point HF or HCl?
Hence the intermolecular force between the molecules of HF compound increases which causes higher melting and boiling points. But in the case of HCl, HBr, and HI, they can’t form a hydrogen bond. Hence their melting and boiling points are lower than HF. Due to the high electronegativity of fluorine hydrogen bonds can be formed between HF molecules.
Why is HF more corrosive than HCl?
HF is corrosive because of its proton; it's corrosive to bones because of its fluoride.HF corrodes glass and HCl doesn't because silicon tetrafluoride is a more stable complex than the highly reactive silicon tetrachloride. It has little to do with whether the acid is weak or strong.
Why does HF have a higher boiling point than hi?
a) The intermolecular bonding for HF is van der Waals, whereas for HCL, the intermolecular bonding is hydrogen. Since the van der Waals bond is stronger than hydrogen, HF will have a higher boiling temperature. Since the covalent bond is stronger than van der Waals, HF will have a higher boiling temperature.
Which has the higher boiling point HF or HCl?
Boiling point of HF is greater than HCl .
Why does HF have a higher boiling point?
Hydrogen bonding is the strongest intermolecular force. Due to strong intermolecular forces, the molecules of HF will be tightly packed in a lattice. Thus, HF will have the highest boiling point since the boiling point of a system depends upon the intermolecular interactions.
Why does HI have a higher boiling point than HCl?
The diffuse electron cloud of the HI molecule allows for more successful intermolecular interactions (i.e. more attractive). So, HI should have a higher boiling point than HCl .
Why is HCl boiling point so low?
There is no H-bonding in HCl. So the boiling point is less. On moving further to HBr and HI, the size of the molecules increases and so the van der Waal's forces increase and so does the boiling point. Hence HCl has the lowest boiling point.
Why does HF have hydrogen bonding but not HCl?
The atom's size, in terms of electronegativity, is such that hydrogen bonds cannot form because the electron density is too low. This is why, whereas \[HF\] exhibits hydrogen bonding, \[HCl\]does not. Because chlorine is a big atom, the \[HCl\]has a high electronegativity value, but not enough to form a hydrogen bond.
Which one has the highest boiling point HCl HF HBr HI?
HFHF has the highest boiling point this is followed by HI, then HBr with HCl having the lowest boiling point of the four molecules. This is because HF is able to form Hydrogen bonds whereas the other three molecules are unable to do so because their electronegativity is not large enough to create a sufficient dipole.
Why is HI a better acid than HCl?
HI has longer bond than HCl, making its bond weaker. Therefore it is easier for HI to lose H+, making it a stronger acid.
Why is HI more reactive than HCl?
HI has the lowest bond dissociation energy due to longer bond length that's why it is most reactive.
Does HF have higher boiling point?
Since the hydrogen bond is stronger than van der Waals, HF will have a higher boiling temperature. Reason: This is because of the electronegativity property of Fluorine. Fluorine is the most electronegative atom.
Why does HF have a higher boiling point than HCL HBR and HI?
HF is hydrogen bonded, thus has highest boiling point, and it is liquid at or below 19oC. The remaining hydrogen halides are gaseous and their boiling points depend on the van der waal's forces. Larger the size (or molecular mass), greater are the van der Waal's forces, hence higher is the boiling point.
Why does HF have a higher boiling point than co2?
Both have about the same molecular weight, but HF is very polar, so HF has the higher boiling point.
Why is the boiling point of HF higher than all of the others when it should be the lowest?
The higher boiling point of hydrogen fluoride is due to the presence of strong hydrogen bonding between HF molecules which leads to the association of HF molecules and requires higher energy for boiling. Was this answer helpful?
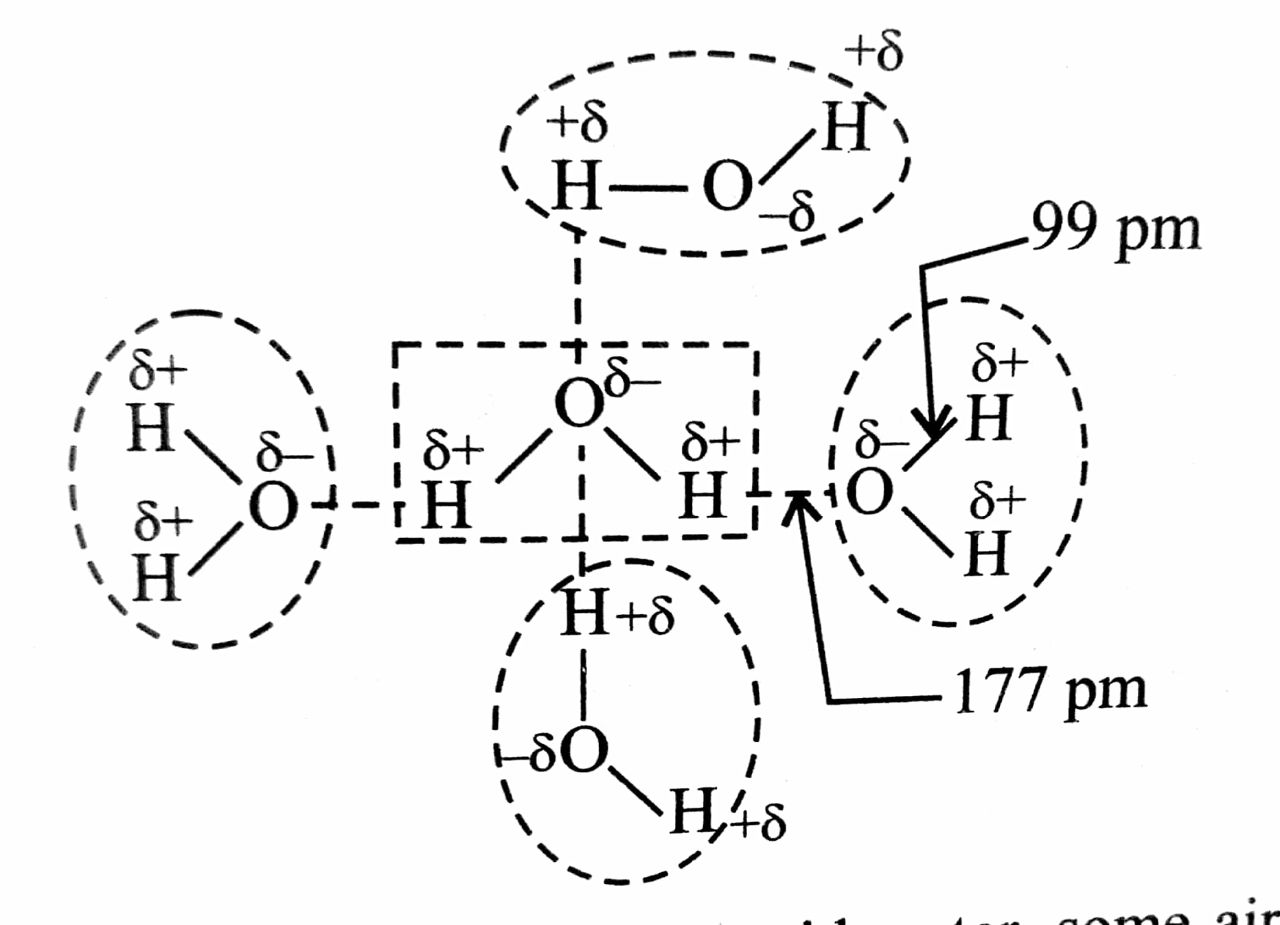
Why does H2O have a higher boiling point than HF?
By the way we all know, why H2O,NH3,HF has significantly higher boiling point than the other period members, yes, because of HYDROGEN BONDING. It is obvious that, more the electronegative element the H attached to, the more will be its magnitude of H Bond. So it the hydrogen-bond strength follows order HF> H2O>NH3. Still, H2O has higher boiling point than HF, the reason is as follows.
How many hydrogen bonds can a molecule of HF form?
HF is a linear molecule, a molecule of HF can only form 2 hydrogen bonds (as ---H-F---H-F---H-F---). But the shape of water molecule is 'V Shaped' due to the presence of 2 lone pairs (of course F in HF also has lone pairs, but it cant utilize it for H-bond due to its linear shape), and a molecule of H2O can form 4 hydrogen bonds (where HF there is only 2). Thus eventhough bond strength is high in HF, the combined bond strengths of 4 Hydrogen Bonds in H2O will overcome the combined bond strength of 2 Hydrogen bonds in HF. Thus H2O has more boiling point than HF...
Why is fluorine more electronegative than HCl?
Because of hydrogen bonding. Fluorine is the most electronegative element so the electrons shared in the H-F covalent bond spend more time around the F atom than the electrons in the H-Cl bond do around the Cl atom. Thus there is a larger dipole in HF than in HCl so there are larger electrostatic forces to be overcome in boiling HF than in HCl.
Why does hydrogen fluoride pull together?
In application, hydrogen fluoride will pull together due to hydrogen bonding, and more energy is required to separate the molecules to transition them from a liquid to a gas. The absence of the hydrogen bonding in hydrogen chloride means that it’s easier to pull the molecules apart.
What is the only hydrogen halide that forms hydrogen bonds?
HF is actually the only hydrogen halide that forms Hydrogen bond s. After that, inter-molecular bonding occurs through Van der Waals forces which -- increase as the number of electrons increase within an atom.
Which has more dispersion force, HCl or HBr?
Now, you need to determine their London Dispersion Forces- HBr is bigger than HCl (it is lower on the periodic table), so it has much more dispersion force.:)
Why does the boiling point increase as the boiling point goes down?
The order of boiling point down the group increases because of the increase in London dispersion forces and it becomes the major factor because as go down the group there is big jump in the molar mass .
Which has the highest boiling point?
WATER has the highest boiling point because water is a strong dipole and the molecules are interconnected by hydrogen bonds.
Which has a lower melting point, HF or HI?
Although HI has a higher molecular mass than HF, the Van Der Waal's forces of attraction in HI are overpowered by intermolecular hydrogen bonding in HF. Hence, HF has a lower melting point than HI.
Why does boiling point increase in group 17?
So in the Group 17, the boiling points should increase while going from F to I, but F has the highest boiling point due to ‘hydrogen bonding'.
Why is HCl the lowest compound?
Lowest: HCl because it has the smallest London force of all compound lacking hydrogen bonding.
What is the boiling point of oxygen?
Actually, the two boiling points are very close together at -183 C for oxygen and -191.5 C for carbon monoxide. Differences that are so small mean that there is no dominant “macroscopic” factor that is responsable and it is actually almost balancing.
Why do ions become polarized?
Because, as the size of anion increases, the distance between its nucleus and outermost electrons increases and hence the nucleus can attract the electrons less. So the cation can more easily deform the anion. Therefore in any group in the periodic table the tendency of the anion to be polarized increases from top to bottom. For example the radii of the halide ions are as follows.
Is the trend observed for butyl bromides dependent on the OH group alone?
Note that these trends are not dependent on the OH group alone. For example, the trend observed for the butyl-bromides is more or less the same: