Why is phase diagram for water different?
Water has an unusual phase diagram: its melting point decreases with increasing pressure because ice is less dense than liquid water. The phase diagram of carbon dioxide shows that liquid carbon dioxide cannot exist at atmospheric pressure. Consequently, solid carbon dioxide sublimes directly to a gas.
What makes phase diagram of water different from most phase diagrams of other substances?
Unlike carbon dioxide and most other substances, the phase diagram of water shows a negative slope for the boundary line between the liquid and solid state. This difference has to do with that fact that water actually expands as it goes from the liquid state to the solid state.
Why water has a curve with a slope opposite that of most substances?
As you increase the pressure, the density of a substance increases. Thus, the negative slope indicates that the liquid phase has a greater density than the solid phase. In other words, the density of ice is less than that of water. For most other common substances, the solid is denser than the liquid.
What is the phase diagram of water?
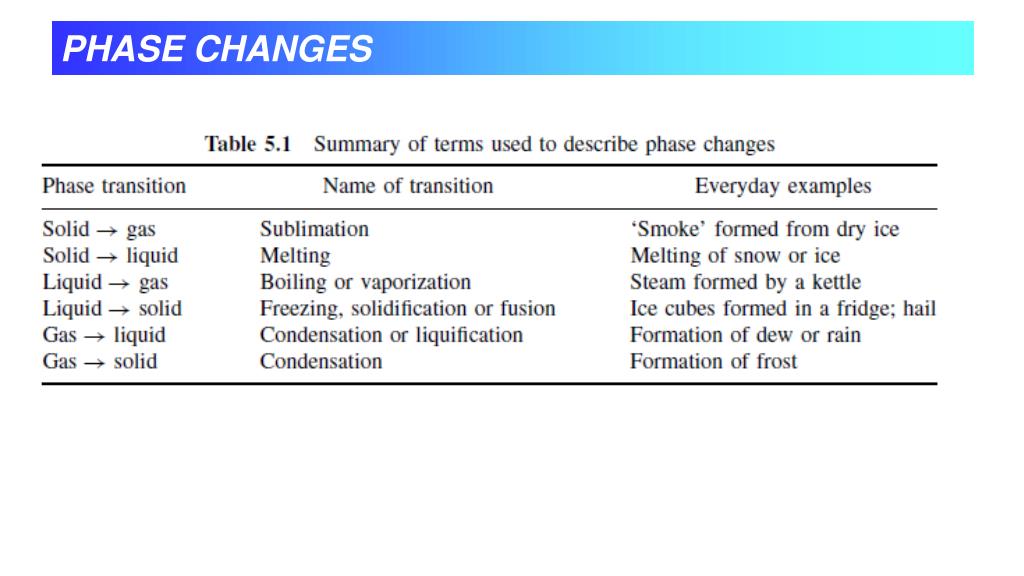
The water phase diagram is a pressure-temperature diagram that represents various water phases, including water, steam, and ice, under different conditions. The phase diagram is known to include phase boundaries.
How water's phase diagram differs from those of most substances What property of water causes the difference?
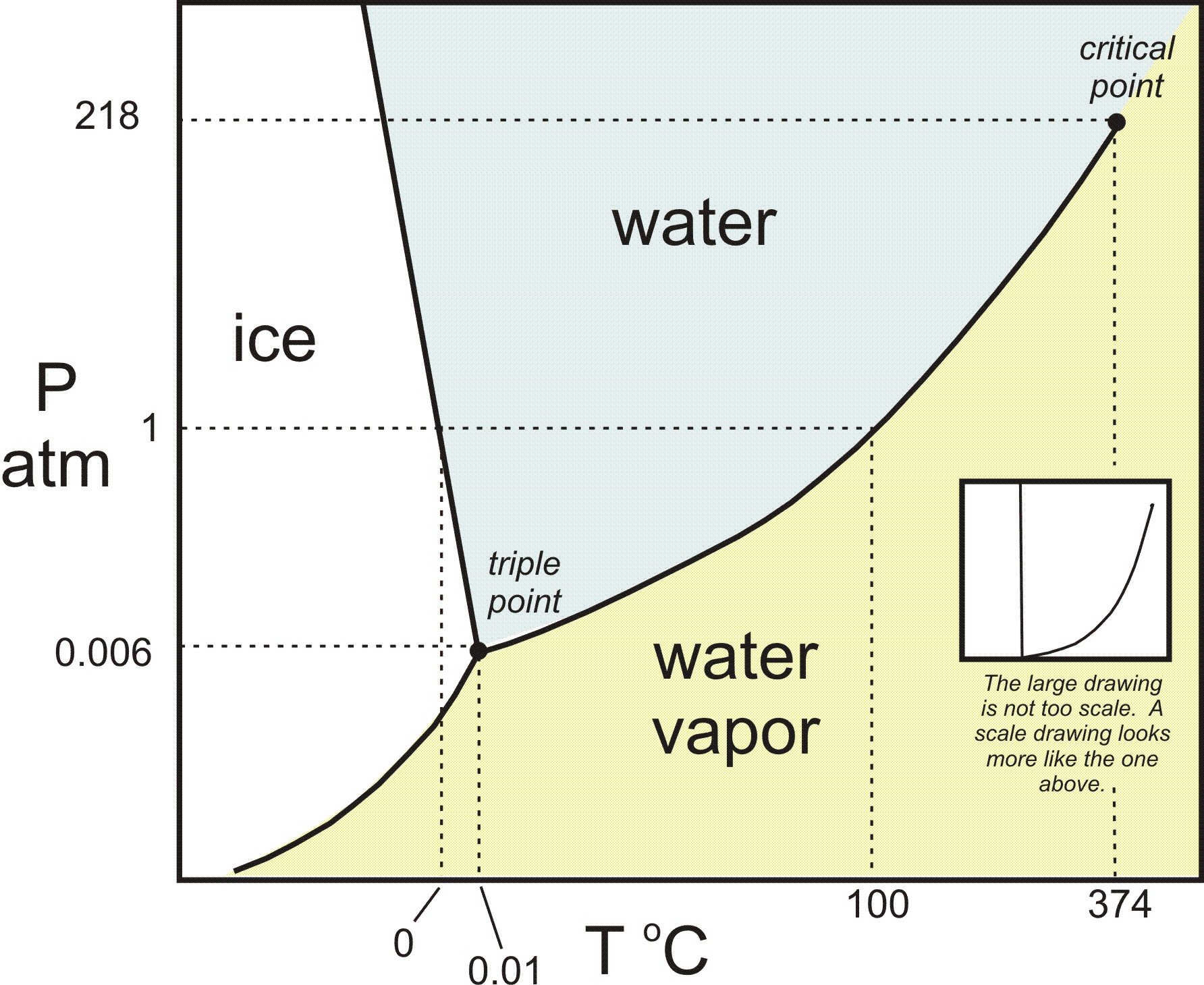
The phase diagram for water differs from the phase diagrams for most other substances. The melting curve for most substances has a positive slope; however, the melting curve for water has a negative slope.
What is the difference between phase diagram of water and phase diagram of carbon dioxide?
The Phase Diagram of Carbon Dioxide In contrast to the phase diagram of water, the phase diagram of CO2 (Figure 12.4. 3) has a more typical melting curve, sloping up and to the right. The triple point is −56.6°C and 5.11 atm, which means that liquid CO2 cannot exist at pressures lower than 5.11 atm.
What makes water an exception to the phase change rule?
What makes water an exception to the phase change rule? Water expands when it freezes; thus, its molecules get farther apart. All other natural substances contract when they freeze because their molecules get closer together.
Do all substances have the same phase diagram?
You can draw phase diagrams for most substances, but the materials in each phase aren't necessarily the same substance.
Where is the critical point in a phase diagram for water?
In water, the critical point occurs at 647.096 K (373.946 °C; 705.103 °F) and 22.064 megapascals (3,200.1 psi; 217.75 atm). In the vicinity of the critical point, the physical properties of the liquid and the vapor change dramatically, with both phases becoming even more similar.
What is the purpose of a phase diagram?
phase diagram, graph showing the limiting conditions for solid, liquid, and gaseous phases of a single substance or of a mixture of substances while undergoing changes in pressure and temperature or in some other combination of variables, such as solubility and temperature.
How many phases of water are represented in its phase diagram?
three phasesAt the pressure and temperature of the triple point, all three phases (solid, liquid and gas) exist in equilibrium. The triple point for water occurs at a pressure of 4.6 torr and 0.01oC.
How many phases are there in water system?
The water system: The three phases are liquid, ice and vapour.
What feature of the phase diagram makes water so special?
In water's diagram, the slope of the line between the solid and liquid states is negative rather than positive. The reason is that water is an unusual substance in that its solid state is less dense than the liquid state.
What are the most important differences between the phase diagram of a pure solvent and the phase diagram of a solution of that solvent?
Two differences we can see on phase diagrams of a pure solvent and a solution are changes in. Solution will always have a higher boiling point \textbf{higher boiling point} higher boiling point and lower freezing point \textbf{lower freezing point} lower freezing point (check pages 501, 502).
Do all substances have the same phase diagram?
You can draw phase diagrams for most substances, but the materials in each phase aren't necessarily the same substance.
What can you say about the phase diagram What can you say about water?
There is one significant difference between the general phase diagram and the water phase diagram. The slope of the line connecting the solid and liquid states in the water diagram is negative rather than positive. Water is an unusual substance in that its solid state is less dense than its liquid state.
What is the difference between the general phase diagram and the phase diagram for water?
The slope of the line connecting the solid and liquid states in the water diagram is negative rather than positive.
Define critical temperature.
A substance’s critical temperature (T C ) is the highest temperature at which it can exist as a liquid.
What does the critical point mean in the phase diagram of water?
The critical point is the point at which the critical temperature and pressure meet. Water particles in the gas phase are moving very quickly at 37...
Why does the melting point of ice get lower at higher pressures?
When pressure increases, the melting point of ice decreases because volume decreases, and the volume of water is less than that of ice.
Is it possible to compress steam to form a liquid if the temperature is higher than 374°C?
Water particles in the gas phase are moving very quickly at 373.99°C. No matter how much pressure is applied to the gas, the gas phase cannot lique...
What is the phase diagram of water?
The phase diagram of water is a representation of the various pressure and temperature combinations that create each phase. The three phases are so...
How many curves are there in a phase diagram of water?
There are two curves on the phase diagram of water. The two curves, or lines, indicate the areas of solid, liquid and gas. At one temperature-press...
Why is the phase diagram of water different?
The phase diagram of water is different than other phase diagrams. This is because the density of water as a solid is less than the density of liqu...
Why is it important to compare water phase diagrams?
It also might be a good idea to compare the water phase diagram to another substance, because who knows what they'll have you looking for next on Planet Z. If you compare water to other substances, you'll notice a couple of differences.
What Is a Phase Diagram?
A phase diagram shows the state a substance is in at different pressures and temperatures.
Why do scientists use phase diagrams?
Lesson Summary. Scientists use phase diagrams to organize a lot of information on one graph. It's handy to know at what temperature and pressure a substance will be a liquid, solid or gas, as you saw when you figured out there was no liquid water on Planet Z. Real-life scientists used a phase diagram for water to determine if there was liquid water ...
What happens when a solid becomes a gas?
Think of the dew on the leaves. The gas in the air cooled, and some of it turned into liquid, or dew. This last phase change is probably less familiar: when a solid becomes a gas, or sublimation. If you've ever seen dry ice, or solid carbon dioxide, you've probably watched it turn directly into a gas.
Why does water vapor leave noodles?
That is why you see water vapor leaving your pot as you boil noodles. A lot of that water leaves the pot as water vapor, a gas. The reverse of this is condensation. This is when a gas turns into a liquid. Think of the dew on the leaves. The gas in the air cooled, and some of it turned into liquid, or dew.
What is phase change?
Well, those are all phase changes, or the change from one state to another state. In this context, the word 'state' refers to a substance's form: solid, liquid or gas. Let's take a moment to talk about phase changes and give some examples. When a solid changes into a liquid, melting has occurred.
What happens when a solid changes into a liquid?
Let's take a moment to talk about phase changes and give some examples. When a solid changes into a liquid, melting has occurred. Just think about an ice cube melting in a glass. Conversely, when a liquid changes into a solid, freezing has occurred. That's what happened to those frozen puddles.
Water Phase Diagram
A phase diagram is a graphic illustration that relates the pressure and temperature combinations responsible for the solid, liquid and gas phases of a substance. Sometimes known as a pressure-temperature or PT diagram, this representation can be very useful in determining what to expect when temperature and/or pressure conditions change.
Compare the Density of Water in Its Three Phases
Density is defined as the amount of matter in a given volume. The formula for calculating density is {eq}Density = \frac {Mass} {Volume} {/eq}
What is the pressure indicated in the phase diagram?
The pressure indicated in the phase diagram is the external pressure.
Does a mixture behave as two separate materials?
A mixture does not behave as two separate materials that just happen to be located on top of each other. That would ignore the interaction between them, which in general severally interferes with their combined phase diagram and with their combined properties.
Does a phase diagram apply to another material?
Neither nor. If other liquids/gases/substances are present (if you have a mixture), then you basically have another material. Then this phase diagram doesn' t apply. Another material has another phase diagram.
Is a mixture a separate material?
A mixture does not be have as two separate materials that just happen to be located on top of each other. That would ignore the interaction between them, which in general severally interferes with their combined phase diagram and with their combined properties. That is why for example the tensile strength of a cobber-nickel alloy is larger than the tensile strength of either pure cobber or pure nickel rather than a value in between.
What is the phase diagram of water?
We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. For example, a pressure of 50 kPa and a temperature of −10 °C correspond to the region of the diagram labeled “ice.” Under these conditions, water exists only as a solid (ice). A pressure of 50 kPa and a temperature of 50 °C correspond to the “water” region—here, water exists only as a liquid. At 25 kPa and 200 °C, water exists only in the gaseous state. Note that on the H 2 O phase diagram, the pressure and temperature axes are not drawn to a constant scale in order to permit the illustration of several important features as described here.
How to describe a phase diagram?
By the end of this section, you will be able to: 1 Explain the construction and use of a typical phase diagram 2 Use phase diagrams to identify stable phases at given temperatures and pressures, and to describe phase transitions resulting from changes in these properties 3 Describe the supercritical fluid phase of matter
What temperature does the liquid vapor curve end?
Notice that the liquid-vapor curve terminates at a temperature of 374 °C and a pressure of 218 atm, indicating that water cannot exist as a liquid above this temperature, regardless of the pressure. The physical properties of water under these conditions are intermediate between those of its liquid and gaseous phases.
What is the effect of the pressures beneath glaciers on glaciers?
The immense pressures beneath glaciers result in partial melting to produce a layer of water that provides lubrication to assist glacial movement . This satellite photograph shows the advancing edge of the Perito Moreno glacier in Argentina. (credit: NASA)
Why does a pressure cooker cook faster?
Pressure cookers allow food to cook faster because the higher pressure inside the pressure cooker increases the boiling temperature of water. A particular pressure cooker has a safety valve that is set to vent steam if the pressure exceeds 3.4 atm. What is the approximate maximum temperature that can be reached inside this pressure cooker? Explain your reasoning.
Why use phase diagrams?
Use phase diagrams to identify stable phases at given temperatures and pressures, and to describe phase transitions resulting from changes in these properties
Which element has one gas phase, one liquid phase, and two solid phases?
Explain your answers. Elemental carbon has one gas phase, one liquid phase, and two different solid phases, as shown in the phase diagram: (a) On the phase diagram, label the gas and liquid regions. (b) Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase.