Why does H2O have a higher boiling point than HF?
Still, H2O has higher boiling point than HF, the reason is as follows. HF is a linear molecule, a molecule of HF can only form 2 hydrogen bonds (as ---H-F---H-F---H-F---). But the shape of water molecule is 'V Shaped' due to the presence of 2 lone pairs (of course F in HF also has lo
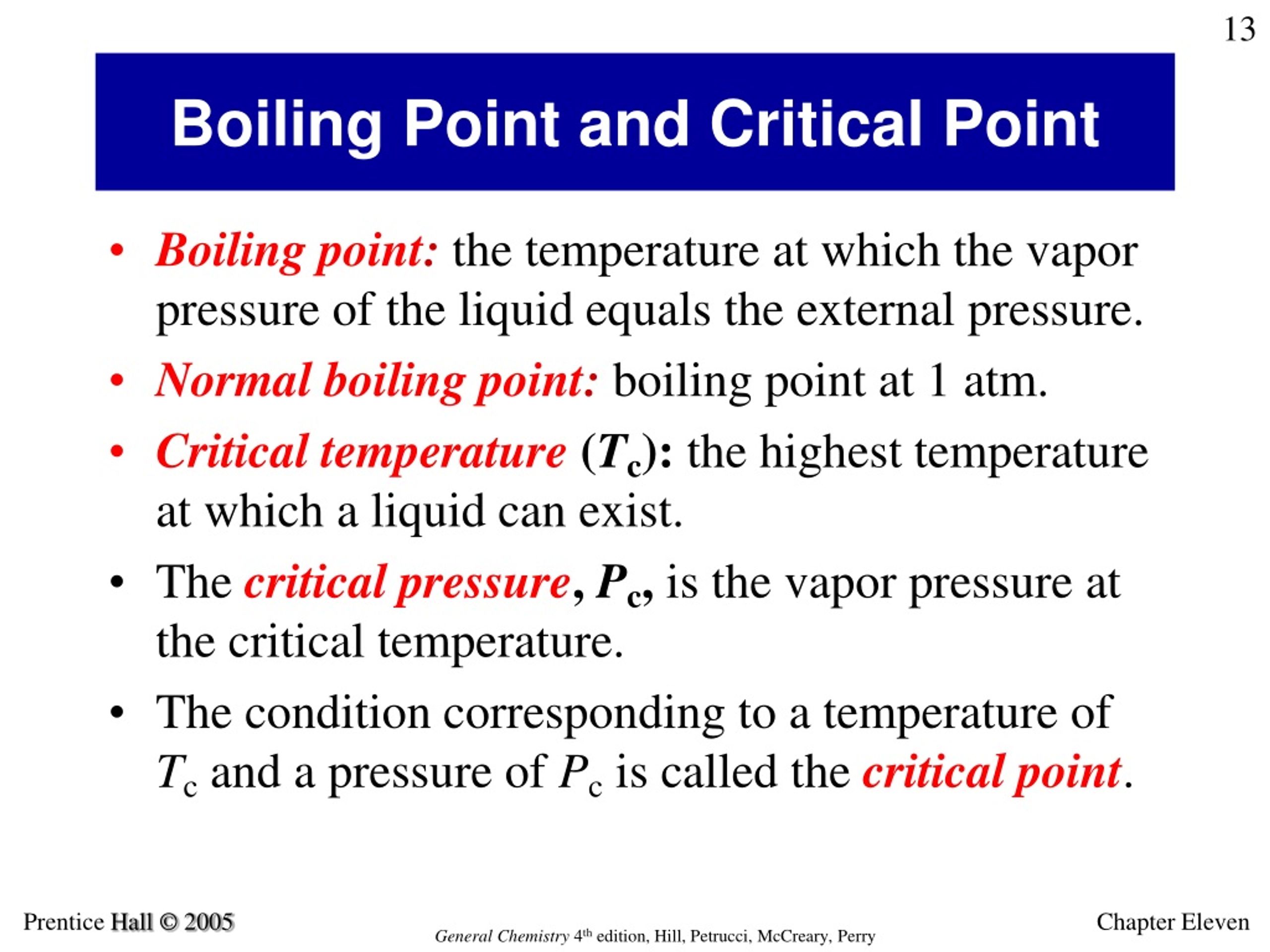
Does water have a higher boiling point than other liquids?
Of course yes, water has a higher boiling point due to intermolecular forces of attraction (hydrogen bonds) which actually requires a lot of energy to be Have you ever googled yourself?
Why does H2O have the highest melting point and H2S has lowest?
Why does H2O have the highest and H2S has the lowest value of melting and boiling points? In H2O, H2 is bind with oxygen which Is more electro negative therefore polarity develops on H2O…..and hydrogen bonding occur between the water molecule…..Since hydrogen bonding is very strong..so large amt of energy is required to break this bond……
Is the boiling point of water simply because of H-bonds?
Is this simply because of the energy required to break H-bonds? Yes, the “unexpectedly high” boiling point of water, for its size, is due the strong intermolecular forces between water molecules (hydrogen bonds). The same is true for HF and NH3 compared to their larger group members.
Does H2O have the highest boiling point?
4:235:18Why is the Boiling Point of water (H2O) so high? - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo to summarize the boiling point of water is so high because water molecules have hydrogen bondingMoreSo to summarize the boiling point of water is so high because water molecules have hydrogen bonding with each other among other intermolecular forces hydrogen bonding is the strongest.
Which has the highest boiling point and why?
The chemical element with the lowest boiling point is Helium and the element with the highest boiling point is Tungsten.
How do you determine what has the highest boiling point?
Explanation: Boiling point is highly dependent on the intermolecular forces of a compound. Compounds with stronger intermolecular forces, larger masses, and less branching will have higher boiling points.
Which liquid has the highest boiling point?
1) CH3COOH has the highest boiling point. The strength of intermolecular forces present in a molecule determines the boiling point of a molecule. CH3COOH has the highest boiling point because it has hydrogen bonds.
Which has the higher boiling point H2O or H2S Why?
Which out of H2O and H2S has higher boiling point and why ? Water has a higher boiling point than hydrogen sulphide. The intermolecular attractions between water molecules are stronger than H2S molecules due to hydrogen bonding in H2O due to high electronegativity and small size of oxygen atom.
Which has the highest boiling point quizlet?
I2, has the highest boiling point because it has the highest molar mass. Since the halogens are all similar in other ways, we expect I2 to have the greatest dispersion forces and therefore the highest boiling point (and in fact it does).
Which of the following has higher boiling point and why 0.1 m nacl or 0.1 M glucose?
Solution : 0.1 molal solution of sodium chloride will have higher `DeltaT_(b)` and higher boiling point as well because it dissociates into ions. At the sametime, 0.1 molal glucose solution being a molecular solid will not dissociate into ions.
Which compound will have the highest boiling point ch4 ch3ch3 CH3CH2OH ch3ch3?
CH3CH2OH has more dispersion forces than CH3OH, so it has the highest boiling point.
Why is the boiling point of H2O higher than that of H2S?
It's due to the higher electronegativity of Oxygen than Sulphur that it makes Intermolecular Hydrogen bonds with the Hydrogen Atoms of Other water molecules thus the Boiling Point of H2O increases and is much higher than that of H2S.
Why is H20 a high boiling point?
The reason that h20 has a high boiling point is because of an intermolecular force called hydrogen bounding ( which is separate from the OH bonds) When water is boiled the OH bonds are not broken, the water molecules just move away from one another enough that they become a gas.
How is hydrogen bond formed?
Hydrogen bond is formed between two molecules if they have hydrogen and any of the three electronegative atoms (N,O,F) covalently bonded to each other . As there is no (NOF) in H2S , there is no hydrogen bond there although it has dipole dipole forces.
What is the difference between a water molecule and an oxigen molecule?
The two molecules has the same geometrical form, the only difference is the smaller and higher electronegativity O atom. Due to its higher electronegativity, The electrons of the H atoms spend more time around the oxigen, making slightly dipole the whole molecule. The "positive" side, with the hidrogens attracted to the other water-molecules oxigen atoms, creating a weak H- or Van der Waals bond. These connected water molcules creating tangled water chains and required more energy to break apart.
Why does the angle of the bond increase in H2O?
Consequently more bond pair- bond pair repulsion take place,so bond angle get increase. On the other hand in H2S, because of less electronegative character of S,less repulsion is present so angle get decrease.
Which molecule has the lowest boiling point?
H2S has the lowest boiling point. As the molar mass increases boiling point of the hydrides increases except H2O. The exception of water is due to intermolecular hydrogen bonding among the water molecules. Water molecules are in associated state. The strong hydrogen bonding is formed due to high electronegativity and extremely smaller size of oxygen. The high ‘O—H’ bond polarity is effective for strong H-bonding. Decreasing order of boiling point: H2O > H2Te > H2Se > H2S.
Which atoms form intermolecular H bonds?
H2S forms intermolecular H bonding as below: In H2O and H2S , Oxygen and Sulphur are the central atoms respectively.Among Oxygen and Sulphur , Oxygen is more electronegative (tendency to gain electrons) and hence it can form more number of intermolecular H bonding with other water molecules than Sulphur in H2S.
Which molecule has more hydrogen bonding than water?
O and H F have electronegative oxygen and fluorine atoms respectively in their molecule. In fact, Fluorine is more electronegative than Oxygen, so technically, H F should engage in more hydrogen bonding than water. But the scenario is different. Each water molecule has two hydrogen atoms. Whereas, each H F molecule has only one hydrogen atom.
How many hydrogen atoms are in water?
Each water molecule has two hydrogen atoms. Whereas, each H F molecule has only one hydrogen atom. And hence, water molecule takes part in extensive and more hydrogen bonding than Hydrofluoric acid. Water molecule through its extensive hydrogen bonding forms a bulky molecule and it is very difficult to break its bonds.