Why is the shape of water molecule non-linear?
In water, the molecule is bent due to an almost 105 degree bond in the angles between the H atoms on either side of the O atom in the center. This causes the water molecule to have a non-linear shape. It contains two pairs of bonded electrons and two unshared lone pairs.
Why is the shape of water Bent?
The reason water has a bent shape is that the two lone pair of electrons are on the same side of the molecule. This repulsion of the lone pairs of electrons on the oxygen atom causes the bond of the hydrogen to the oxygen to be pushed downward (or upward, depending on your point of view). What is the hybridization of bent?
Why is H₂O V shaped?
water - Why is H₂O V shaped? - Chemistry Stack Exchange We know that the molecule of H₂O is V-shaped. This is what makes it a dipole. But why is that? I mean, if the hydrogens have a partial positive charge, then they should try to get away from each ... Stack Exchange Network
Why is the middle of a water molecule negatively charged?
That is because in a water molecule, the middle which is where the oxygen atom is located, is negatively charged and either end which are the hydrogens are positively charged. In water, the molecule is bent due to an almost 105 degree bond in the angles between the H atoms on either side of the O atom in the center.

Are water molecules V-shaped?
The dynamic interactions of water molecules. Individual H2O molecules are V-shaped, consisting of two hydrogen atoms (depicted in white) attached to the sides of a single oxygen atom (depicted in red).
Why are some molecules V-shaped?
Why is CO2 a linear molecule whereas H2O has a v-shaped geometry? This is due to the different numbers of electrons in each molecule and VSEPR (Valence Shell Electron Repulsion) theory. This theory states that as electrons are negatively charged, the valence electrons in different atoms in a molecule repel each other.
What does V-shaped mean in chemistry?
In chemistry, molecules with a non-collinear arrangement of two adjacent bonds have bent molecular geometry, also known as angular or V-shaped. Certain atoms, such as oxygen, will almost always set their two (or more) covalent bonds in non-collinear directions due to their electron configuration.
What makes a molecule that is V-shaped polar?
In order to have a polar molecule, there must be unequal distribution of the negatively charged electrons in the orbitals of the molecule. The dipoles are unequally charged either because of the net electronegativity of the molecule or by the shape of the molecule causing the negative dipole.
Why is h2o bent and not linear?
The molecule adopts a bent structure because of the two lone pairs of electrons on the oxygen atom.
Why is h20 not symmetrical?
It's because of the lone pairs of electrons, yes. There are 4 pairs of electrons, and only 2 are used in bonding. The 4 pairs on the central atom arrange themselves into a tetrahedral shape. Only two of these are bonds, so the molecule is in the V-shape you see.
Which molecule is V shape?
In SnCl2,due to repulsion between lone pair and bond pair,the angle is less than 120o and have V-shape.
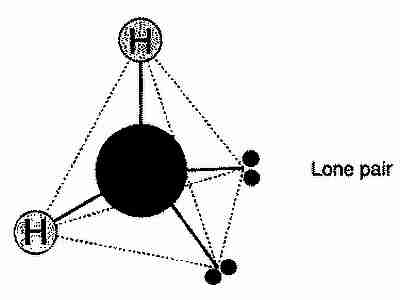
What is the shape of water molecule?
The molecular shape is an almost symmetrical tetrahedron with oxygen positioned in its centre. The corners are occupied by two hydrogen atoms (H) and by the two pairs of electrons (grey) of oxygen (O) that not involved in chemical bonding (lone pairs).
Why is an oxygen atom actually V shaped?
The “V” shape arises from the arrangement of electrons in the molecule, which causes an imbalance in electrical charge, with the oxygen point of the “V” slightly more negative than the opposite end near the hydrogen atoms.
Why is water a polar molecule?
Water is a Polar Covalent Molecule The unequal sharing of electrons between the atoms and the unsymmetrical shape of the molecule means that a water molecule has two poles - a positive charge on the hydrogen pole (side) and a negative charge on the oxygen pole (side).
What determines molecular shape?
The shape of a molecule is determined by the location of the nuclei and its electrons. The electrons and the nuclei settle into positions that minimize repulsion and maximize attraction. Thus, the molecule's shape reflects its equilibrium state in which it has the lowest possible energy in the system.
How does the shape of a molecule affect its function?
Molecular shape is crucial in biology because of the way it determines how most molecules recognize and respond to each other. One nerve cell in the brain signals another by releasing molecules of a specific shape to go find matching receptor molecules on the surface of the receiving cell.
What is the shape of a water molecule?
When it comes to molecular shapes, there are two different terms. Electron domain geometry and molecular geometry. Electron domain geometry of the water molecule is tetrahedral whereas the molecular geometry of the water molecule is bent (or angular.)
How is the shape of a molecule determined?
Jokes aside, the shape of a molecule is determined by the VSEPR theory (Valence Shell Electron Pair Repulsion.) Molecules arrange themselves in such a way that they minimize the potential energy. High energy molecules are unstable compared to low energy molecules.
How many valence electrons does water have?
Water has oxygen as its cetral atom whose valence electrons is 6. In the molecular formula of water we see that it has bonded to 2 other atoms which is 2 atoms of hydrogen.2 out of 6 electrons are shared with hydrogen. So there are still two lone pairs remaining on the oxygen atom which is not bonded. Because of its presence it pushes the hydrogen atoms away due to repulsion and creates a tertrahedral geometry.
Why does water have a non-linear shape?
This causes thewater molecule to have a non-linear shape. It contains two pairs of bonded electrons and two unshared lone pairs. Due to itsshape, and polarity, water molecules are greatly attracted to one another. Water molecule attract towards the gravity so, it stretches towards the earth and takes the shape like’V’
What is the stability of an ion in water?
The stability of an ion in water is related to its ionization energy. The ionization energy (Ei) is qualitatively defined as the amount of energy required to remove the most loosely bound electron, the valence electron, of an isolated gaseous atom to form a cation.
How to understand geometry from Molecular Orbital point of view?
To understand geometry from Molecular Orbital point of view, one must calculate MO energies for linear (180⁰) and bent (90º) geometries. Then, lines correlating MO energies of linear and bent molecules must be drawn. Resulting graphical representation of variation of MO energy with bond angle is know as Walsh diagram.
What is nonpolar covalent?
Nonpolar covalent compounds are the compounds with equal sharing of electrons.
Why is CO2 a linear molecule whereas H2O has a v-shaped geometry?from mytutor.co.uk
Why is CO2 a linear molecule whereas H2O has a v-shaped geometry? This is due to the different numbers of electrons in each molecule and VSEPR (Valence Shell Electron Repulsion) theory. This theory states that as electrons are negatively charged, the valence electrons in different atoms in a molecule repel each other.
Why can't we put hydrogen in the centre?from mytutor.co.uk
We cannot put hydrogen in the centre because it can only hold two electrons, due to its principle quantum number of 1. Therefore oxygen goes in the centre. Forming single bonds to each hydrogen leaves two more pairs of electrons which go around the oxygen atom, to complete the octet. These are lone pairs.
Which type of electron takes up more space than a bonding electron?from mytutor.co.uk
But, lone pair electrons take up more space than bonding electrons, as they are only attracted to one atom rather than two, so they repel more than bonding electron. Therefore we can order repulsions between different types of electron pairs: lone pair-lone pair > bonding pair- lone pair > bonding pair - bonding pair.
Why is water polar?
That is because in a water molecule, the middle which is where the oxygen atom is located, is negatively charged and either end which are the hydrogens are positively charged . In water, the molecule is bent due to an almost 105 degree bond in the angles between the H atoms on either side of the O atom in the center. This causes the water molecule to have a non-linear shape. It contains two pairs of bonded electrons and two unshared lone pairs. Due to its shape, and polarity, water molecules are greatly attracted to one another. This creates surface tension. It also causes a drop of water to seem to stretch out of a faucet before it finally falls.
Is water polar or nonpolar?
Water, which contains two hydrogen atoms and one oxygen atom in a molecule is a polar molecule. That is because in a water molecule, the middle which is where the oxygen atom is located, is negatively charged and either end which are the hydrogens are positively charged. In water, the molecule is ...
Why is it naive to think that water is bent?
It is naïve because the water molecule is a bent water molecule… bent at about 104.5°. It is a good thing for us that this is so, since this imparts a degree of polarity to the water molecule. Polarity, in turn, gives rise to hydrogen bonding. The hydrogen bonding of molecules assures water’s liquidity.
How many hydrogen atoms are in water?
In view of the above, it becomes apparent the structure of water is much more accurately represented as an oxygen atom attached to two hydrogen atoms and two electron pairs. Notice the essentially tetrahedral layout in the image.
Why does hydrogen bonding affect ice?
In addition hydrogen bonding influences water’s crystallization, so that ice is lighter than very cold water. Ice thus floats, forming an insulating blanket atop lakes and other bodies of water. This prevents them from freezing solid, killing all life within those bodies.