
Why are alkali metals named as such?
The alkali metals are named as such because they form alkaline products (i.e. hydroxides) when they react with water. Elements in Group 1 have 1 electron in their outermost shell.
Which of the following metals can be substituted for hydrogen?
Alkali metals can be substituted for hydrogen. Any of them. They include lithim (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs) and francium (Fr). The alkali metals are in group one of the periodic table of the elements, and are all stacked up in the column below hydrogen.
What is hydrogen used for in everyday life?
Whilst hydrogen is commonly found in water and hydrocarbons, its main use as an element is in processing fossil fuels and fats and oils, and in the production of ammonia for food production. The lithium-containing mineral petalite was discovered in 1800 by Jose Bonifacio de Andrada e Silva.
See more

Why is hydrogen located in the alkali metals?
Hydrogen is placed with alkali metals as it has one electron in its valence shell similar to the alkali metals.
Why is hydrogen placed in the first group?
Hydrogen is placed in group one because it has certain properties of group I elements. Hydrogen, like other alkali metals, is a powerful reducing agent. Hydrogen has an outer electronic configuration 1 s 1 that is comparable with those of group I elements.
Is hydrogen considered an alkali metal?
Group 1A (or IA) of the periodic table are the alkali metals: hydrogen (H), lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). These are (except for hydrogen) soft, shiny, low-melting, highly reactive metals, which tarnish when exposed to air.
Why is hydrogen placed?
Hydrogen is placed above group in the periodic table because it has ns1 electron configuration like the alkali metals. However, it varies greatly from the alkali metals as it forms cations (H+) more reluctantly than the other alkali metals.
Why is hydrogen placed in the reactivity series of metals?
Hydrogen is included in the reactivity series of the metals as it resembles alkali metals in some of its properties such as: It looses electron to form positive ion. It combines with non-metals which are electron attracting species. During hydrolysis if any hydride hydrogen gas is liberated at cathode.
Why is hydrogen not a member of the alkali metals?
The alkali metals include: lithium, sodium, potassium, rubidium, cesium, and francium. Although often listed in Group 1 due to its electronic configuration, hydrogen is not technically an alkali metal since it rarely exhibits similar behavior.
Why hydrogen can be placed in group 1 and 17?
The reason why the hydrogen can be placed in both group 1 and group 17 is that it resembles Alkali metals in some of its properties like it can easily form cations so, it can be placed in Group 1 of periodic table but it also resembles Halogens in its properties like it forms H2 which is true property of halogen that ...
Why hydrogen is not considered as an alkali metal or halogen?
The only thing hydrogen has in common with alkali metals is in having one electron in its outermost shell and having a valency (combining power) of one. Unlike the group one elements hydrogen is clearly not a metal (it is a gas at room temperature) and is a poor conductor of heat and electricity.
Why hydrogen is placed in group 1 or group 17 in the first period?
The reason why the hydrogen can be placed in both group 1 and group 17 is that it resembles Alkali metals in some of its properties like it can easily form cations so, it can be placed in Group 1 of periodic table but it also resembles Halogens in its properties like it forms H2 which is true property of halogen that ...
Why hydrogen can be placed in group 1 and 7?
Assertion :Hydrogen can be placed in both I A group and VII A group of the modern periodic table. Reason: Similarity in properties with both IA and VII A group.
Why has hydrogen been placed in the first group of the periodic table although it is a non metal?
In the modern periodic table, hydrogen(H) is placed in group I and this is mainly because it resembles certain properties of group I elements, such as: Hydrogen acts as a strong reducing agent like other alkali metals. The outer electronic configuration of hydrogen (1s1) is similar to group I elements.
Why is hydrogen placed alone in the periodic table?
Solution : Hydrogen possesses unique properties which make it stand apart from other elements. Its properties resemble those of alkali metals as well as halogens - it can lose one electron like alkali metals and gain one electron like halogens.
Why are the elements in Group 1 categorised together?
Group 1 contains hydrogen and the alkali metals. The alkali metals are named as such because they form alkaline products (i.e. hydroxides) when they react with water.
How long does francium last?
Francium is extremely rare and highly unstable; the most stable isotope has a half-life of 22 minutes. It has no known uses.
Why was lepidolite named rubidus?
They named the element after the Latin rubidus, meaning deep red, to reflect the bright red lines in its emission spectrum.
What is sodium used for?
Sodium is also used in medicines and in the production of various organic compounds. Sodium vapour lamps are used in (yellow-coloured) street lighting. Sodium metal itself has few direct uses owing to its high reactivity, with its use as a coolant in some nuclear reactors being one example.
What are the uses of lithium?
Uses: There are many uses for lithium, including in glass and ceramics, lubricants, batteries, as a rocket propellant, and in the production of nuclear energy and weapons. Lithium salts are also used as psychiatric medication.
How many electrons are in group 1?
Elements in Group 1 have 1 electron in their outermost shell. They form cations with a charge of +1, and reactivity increases down the group as the valence electron becomes more easily removed due to a weaker attraction to the nucleus. Group 1 and group 2 together form the s-block due to the valence electrons occupying an s orbital.
What is hydrogen released in?
Hydrogen is released in reactions between acids and metals, and was identified as a distinct substance in these reactions by Henry Cavendish in 1766. Cavendish named the gas inflammable air. In 1781, he found that water was produced when hydrogen was burned in oxygen. Two years later Antoine Lavoisier named the element hydrogene after the Greek hydro- meaning water, and -genes meaning to create or produce.
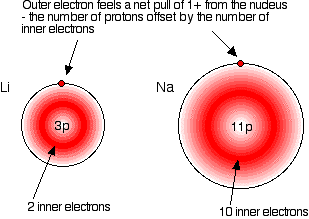
Why Are The Elements in Group 1 categorised Together?
What Elements Are in Group 1 – Hydrogen and Alkali Metals?
- Click on the following elements to learn more about them: 1. Hydrogen 2. Lithium 3. Sodium 4. Potassium 5. Rubidium 6. Caesium 7. Francium
Hydrogen – H – Element 1
- History:
Hydrogen is released in reactions between acids and metals, and was identified as a distinct substance in these reactions by Henry Cavendish in 1766. Cavendish named the gas inflammable air. In 1781, he found that water was produced when hydrogen was burned in oxygen. Two year… - Uses:
Whilst hydrogen is commonly found in water and hydrocarbons, its main use as an element is in processing fossil fuels and fats and oils, and in the production of ammonia for food production.
Lithium – li – Element 3
- History:
The lithium-containing mineral petalite was discovered in 1800 by Jose Bonifacio de Andrada e Silva. In 1817, Johan August Arfwedson identified that it contained a new element as it produced similar alkaline compounds to sodium and potassium, but which were less soluble. The alkaline … - Uses:
There are many uses for lithium, including in glass and ceramics, lubricants, batteries, as a rocket propellant, and in the production of nuclear energy and weapons. Lithium salts are also used as psychiatric medication.
Sodium – Na – Element 11
- History:
Sodium compounds such as salt (NaCl), soda (Na2CO3) and lye (NaOH) have been known for millennia, and used domestically in food, medicines, soap making and various other applications. However, as it is highly reactive, sodium metal is not found naturally. It was first isolated in 1807 … - Uses:
Sodium compounds are primarily used for de-icing (e.g. of roads) and in food (e.g. as salt or in baking). Sodium is also used in medicines and in the production of various organic compounds. Sodium vapour lamps are used in (yellow-coloured) street lighting. Sodium metal itself has few d…
Potassium – K – Element 19
- History:
Potash, i.e. the ash from burning plants and wood, was a well-known source of potassium salts. In 1797, Martin Klaproth discovered potash in the minerals leucite and lepidolite and concluded that it was not produced by plants themselves but must be a material associated with an undiscover… - Uses:
Potassium is mainly used in salts utilised as fertilisers in agriculture.
Rubidium – RB – Element 37
- History:
Robert Bunsen and Gustav Kirchhoff discovered rubidium in 1861. They were using flame spectroscopy to study the mineral lepidolite and identified unique spectral lines which they attributed to a new element. They named the element after the Latin rubidus, meaning deep red… - Uses:
The most common use of rubidium is in fireworks and in atomic clocks. Rubidium vapour is also used in very specific physics applications such as laser cooling and Bose-Einstein condensates.
Caesium – CS – Element 55
- History:
Robert Bunsen and Gustav Kirchhoff discovered caesium in a sample of mineral water using spectroscopy in 1860. They observed bright blue lines in its emission spectrum and concluded there was the presence of a new element. They named it caesium after the Latin caesiusmeanin… - Uses:
Currently, the main use of caesium is in fluids used in oil drilling. Caesium is also used in high precision atomic clocks. It was previously used in vacuum tubes to react with any air that entered the tube and hence maintain a vacuum, but vacuum tube technology is mostly obsolete.
Francium – Fr – Element 87
- History:
The existence of francium was predicted in the late 1800s and the tentative name eka-caesium was used. Francium is highly radioactive and only occurs naturally in extremely small quantities, which led to several false claims of discovery. Radioactive potassium-40 was mistaken for elem… - Uses:
Francium is extremely rare and highly unstable; the most stable isotope has a half-life of 22 minutes. It has no known uses.
Struggling with Chemistry?
- At Matrix, our specialised HSC Chemistry teachers, comprehensive resources and engaging lessons will help boost your marks and confidence! Learn more. © Matrix Education and www.matrix.edu.au, 2022. Unauthorised use and/or duplication of this material without express and written permission from this site’s author and/or owner is strictly prohibited. Excerpts and lin…