
Kinetic Molecular Theory
- Kinetic Molecular Theory and Gas Laws. Kinetic Molecular Theory explains the macroscopic properties of gases and can be used to understand and explain the gas laws.
- Distribution of Molecular Speeds and Collision Frequency. ...
- Root-Mean-Square Speed. ...
What are the five postulates of the kinetic molecular theory?
- The particles are point charges and have no volume: Then, it should be possible to compress the gases to zero volume. ...
- Particles are independent and do not interact: Particles do interact depending upon their nature. ...
- Particles collisions are not elastic: Particle collisions are elastic and they exchange energy. ...
What are some examples of kinetic molecular theory?
Some postulates of kinetic molecular theory: Gases are composed of a large number of particles that behave like hard, spherical objects in a state of constant, random motion. These particles move in a straight line until they collide with another particle or the walls of the container.
What is the postulate of kinetic molecular theory?
The kinetic-molecular theory of gases can be stated as four postulates: A gas consists of molecules in constant random motion. Gas molecules influence each other only by collision; they exert no other forces on each other. All collisions between gas molecules are perfectly elastic; all kinetic energy is conserved.
What are some examples of kinetic theory?
What are some examples of kinetic molecular theory? The molecules obey Newtonian mechanics . The examples of kinetic theory include Brownian Motion- the random movement of dust particles because of collisions with “air” molecules and how gases behave i.e. Boyle’s, Charles’, and Gay-Lussac’s Laws.
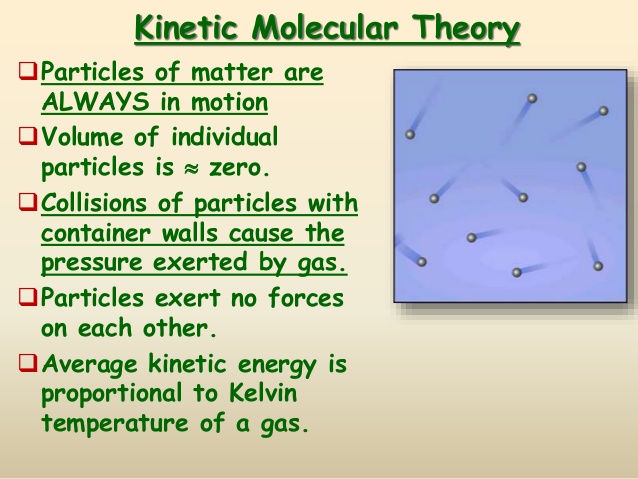
What is most important about kinetic molecular theory?
The kinetic theory of matter helps us understand why matter occurs in various stages (i.e. solid, liquid and gas) and how matter can change from one stage to the next. The kinetic theory of matter also enables one to consider other characteristics of matter.
Why is kinetic molecular theory important to human?
By making several assumptions about the motion and energy of molecules, KMT provides scientists with a useful framework for understanding how the behavior of molecules influences the behaviors of different states of matter, particularly the gas state.
What is the kinetic theory of matter why is it important?
Answer: The kinetic theory of matter helps us to explain why matter exists in different phases (i.e. solid, liquid and gas), and how matter can change from one phase to the next. The kinetic theory of matter also helps us to understand other properties of matter.
How is kinetic molecular theory used in real life?
You can observe a real-life application of Boyle's Law when you fill your bike tires with air. When you pump air into a tire, the gas molecules inside the tire get compressed and packed closer together. This increases the pressure of the gas, and it starts to push against the walls of the tire.
What are the main points of the kinetic theory of matter?
The kinetic theory of matter states that all matter is made of small particles that are in random motion and that have space between them. This means that no matter what phase matter is in, it is made of separate, moving particles.
What are the significance of gases and gas laws in our everyday life?
Amontons' law states that “At constant volume, as the temperature of a gas doubles its pressure also doubles.” Example: It is important to check the pressure of the car tire before heading to a drive. While driving, the temperature of the air in the tire increases and results in flexing.
What does kinetic theory do?
The Kinetic Molecular Theory explains the forces between molecules and the energy that they possess. This theory is based on three theories about matter. Matter is composed of small particles (atoms, molecules, and ions).
What is the importance of potential and kinetic energy in our daily life?
The electricity that fuels people's homes is supplied by potential energy turned kinetic, either in the form of an electric plant fueled by coal, a hydroelectric dam, or other source such as solar cells. The coal is stored potential energy at its most inert; it must be burned to translate itself into kinetic energy.
What is the application of kinetic in daily life?
A bicycle or skateboard in motion possesses kinetic energy. Running water has kinetic energy and it is used to run water mills. Moving air has K.E and is used to derives windmills and push sailing boats, similarly, a bullet fired from a gun has kinetic energy and can penetrate into a target because of its K.E.
How can you relate the kinetic molecular theory with the experiment?
0:432:28Science Experiments Involving the Kinetic Molecular Theory - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe pressure as. I add masses to the top. You'll see that the syringe drops again because I'mMoreThe pressure as. I add masses to the top. You'll see that the syringe drops again because I'm increasing the pressure on the syringe in here the molecules are getting squished.
Why do we use Kelvin instead of Celsius?
As a result, if we said that the kinetic energy of a gas was proportional to the temperature in degrees Celsius, the kinetic energy of any gas cooled below the freezing point of water would be negative.
What does KMT mean in gas?
The KMT assumes that the particles in a gas, like very small children, constantly move from place to place in an unpredictable fashion. This assumption is correct. Furthermore, the KMT assumes that these particles travel in straight lines until they bash into something, at which point they turn around and go somewhere else. As is also the case with very small children, this assumption is true.
Is the KMT model perfect?
All theoretical models (including the KMT) only approximate the behavior of the thing being modeled. The approximations that define each model are designed to make the real phenomenon easier to understand and predict. However, no model is perfect, which explains why weather forecasting models usually get the five-day forecast wrong.
Why does kinetic molecular theory work?
It happens because both substances are made out of molecules that are constantly moving. These molecules have energy; one of the fundamental principles of the kinetic molecular theory. The Kinetic Molecular Theory (KMT) is a model used to explain the behavior of matter. It is based on a series of postulates.
Which has more kinetic energy, liquid or gas?
Gases have more kinetic energy than liquids. Liquids have more kinetic energy than solids. When a substance increases in temperature, heat is being added, and its particles are gaining kinetic energy. Because of their close proximity to one another, liquid and solid particles experience intermolecular forces.
What is KMT based on?
It is based on a series of postulates. Some of the postulates of KMT are as follows: Matter is made of particles that are constantly in motion. This energy in motion is called kinetic energy. The amount of kinetic energy in a substance is related to its temperature. There is space between particles.
What is the strength of intermolecular forces?
Strength of intermolecular force is related to the type of intermolecular force, but it is also affected by the amount of kinetic energy in the substance. The more kinetic energy, the weaker the intermolecular forces.
What type of bond is between polar molecules?
Hydrogen bonds occur between polar molecules that contain an oxygen, nitrogen or fluorine atom covalently bonded to a hydrogen atom. The intermolecular attraction happens between the partially negatively charged oxygen, fluorine or nitrogen and the partially positively charged hydrogen of a neighboring molecule.
Kinetic Molecular Theory Definition (KMT)
Kinetic Molecular Theory (KMT) describes the experimentally discovered behavior of particles. KMT is most often referenced in relation to the behavior of gases, but it could also be applied to solids and liquids.
Kinetic Molecular Theory and Gas Laws
Through the use of KMT, a few different laws have been created to help quantify the relationship between the measurable characteristics of gases. The three laws are called the ideal gas law, Charles' law, and Boyle's law. These three laws relate the pressure, volume, temperature, and amount of a gas.
Kinetic Molecular Theory in Real Life
In this activity, students are going to be looking for substances in their normal environment and describing how the kinetic molecular theory would apply. Students should choose two solids and two liquids, describe the motion of the particles in each and draw a picture.
