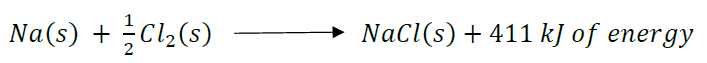
Photosynthesis is considered an endothermic reaction because in this process light energy from sunlight is absorbed to produce oxygen and glucose from carbon dioxide and water in the presence of chlorophyll Chlorophyll is any of several related green pigments found in the mesosomes of cyanobacteria, as well as in the chloroplasts of algae and plants. Its name is derived from the Greek words χλωρός, khloros and φύλλον, phyllon. Chlorophyll is essential in photosynthesis, allowi…Chlorophyll
Redox
Redox is a contraction of the name for chemical reduction-oxidation reaction. A reduction reaction always occurs with an oxidation reaction. Redox reactions include all chemical reactions in which atoms have their oxidation state changed; in general, redox reactions involve the transfer of el…
Why is photosynthesis considered an endergonic?
Is photosynthesis Endergonic or Exergonic What is the energy source that drives it? Photosynthesis is an endergonic reaction. Plants get the required energy—686 kcal to make a mole of glucose—from the environment by capturing light and converting its energy into chemical energy.
Why is NADPH important in photosynthesis?
What is the best supplement for anti aging?
- Curcumin. Curcumin — the main active compound in turmeric — has been shown to possess powerful anti-aging properties, which are attributed to its potent antioxidant potential.
- EGCG.
- Collagen.
- CoQ10.
- Nicotinamide riboside and nicotinamide mononucleotide.
- Crocin.
Why is oxygen not needed in photosynthesis?
Since the concentration of oxygen was negligible, first photosynthesis took place using hydrogen sulfide and organic acid in sea water. However, the level of these materials was not sufficient to continue photosynthesis for long and therefore photosynthesis using water evolved. This type of photosynthesis using water resulted in liberation of oxygen.
Why does photosynthesis require light energy?
Stated another way:
- 100% sunlight → non-bioavailable photons waste is 47%, leaving
- 53% (in the 400–700 nm range) → 30% of photons are lost due to incomplete absorption, leaving
- 37% (absorbed photon energy) → 24% is lost due to wavelength-mismatch degradation to 700 nm energy, leaving
What happens to the energy in an endothermic reaction?
Precisely, in an endothermic reaction energy is absorbed from the environment. During the photosynthesis , the pigments present in the photosynthesizers must absorb the energy of a photon and then use this energy to start a chain of chemical and photochemical events. In contrast, exothermic reactions are reactions that release energy into ...
Why does endothermic reaction heat?
This happens because the energy required to break the existing bonds is greater than the energy released when new bonds are formed.
What are some examples of endothermic reactions?
Other Examples of Endothermic Reaction. -The reaction of crystals from barium hydroxide octahydrate with dry ammonium chloride. -Evaporation of water (water in liquid state is a compound, and heat is absorbed by breaking the bonds in water molecules). -Disolution of ammonium chloride in water.
How do chemical reactions transfer energy?
Chemical reactions transfer energy to, or from, the environment. Endothermic reactions absorb energy from the environment, while exotherms transmit energy to the environment. What determines whether a reaction is endothermic or exothermic, is the balance between the energy that must be supplied to break existing bonds and the energy ...
What is the difference between exothermic and exothermic reactions?
In contrast, exothermic reactions are reactions that release energy into the environment in the form of heat. They feel warm or hot, and can even cause an explosion. In this kind of reaction, the enthalpy change ( amount of energy contained) has a negative value.
Is thermal decomposition endothermic or endothermic?
In addition, any thermal decomposition reaction is endothermic, because the reaction only occurs if heat is introduced into the system.
