Why is salt used in textile dyeing process?
Broadly speaking, Salt is necessary for three ways, firstly, to drive dye into textile during the dyeing process in textile. Secondly, use of salt leads to maximum exhaustion of dye molecules during dyeing process in textiles.
Should I use salt when dyeing my hair?
When using salt, always follow the recommendations of the dye manufacturer, unless you know better.
What is common salt in Reactive dyeing?
Common Salt is a chemical compound belonging to the larger class of salts composed primarily a mineral of sodium chloride (NaCl). The salt added during the reactive dyeing process increases the dye’s attraction to the Cellulosic material. The reactive dyestuff exhaustion is high during dyeing because of adding salt.
Do I need salt for immersion dyeing?
While recipes for direct dye application (like those for tie-dyeing) tend to omit salt, immersion dyeing with acid dyes , as well as lanaset dyes, calls for the use of regular salt (sodium chloride) or Glauber's salt (sodium sulfate decahydrate). PROchem's One Shot dyes do not call for the use of salt, presumably because they already contain it.

Why salt is used in dyeing process?
Salt has an extremely high affinity for water. Broadly speaking, Salt is necessary in three ways, firstly, to drive dye into textile during the dyeing process in textile. Secondly, use of salt leads to maximum exhaustion of dye molecules during dyeing process in textiles.
How does salt affect dye?
In reactive dyeing, the salt increases the affinity of the colorant to the cellulosic substrate. Salt increases reactive dyestuffs' exhaustion rate. As reactive dyestuffs have a lower affinity, more inorganic salt is required in order to accelerate absorption when using reactive dyestuffs.
Why is salt added in the dyeing process of direct dyes?
Addition of salt to the dye bath reduces the solubility of dye in water thus inducing the dye to migrate from water to the fiber core. Therefore both suppression of fiber surface charge and reduction of dye solubility in water help in achieving high exhaustion of dye.
What is the function of salt during dyeing cotton?
In the textile industry, industrial salt is used in the processing of fabrics and materials, such as for dyeing cotton and other cellulose materials. Salt causes the dye to completely penetrate in the fabric when used in a dye bath thereby making the dyeing process uniform and easier.
Which salt is used in dyeing?
Sodium ChlorideGlauber's salt is soluble in water, has a salty, bitter taste and is often used as a mild laxative in medicine; it is also commonly used for dyeing. The generic name of Sodium Chloride (NaCl) is vacuum salt [12,18,19]. Enhancement of the dyestuff affinity. of its solubility.
Why salt is used during dyeing a cotton fabric with reactive dyes?
Salt has an extremely high affinity for water. Broadly speaking, Salt is necessary in three ways, firstly, to drive dye into textile during the dyeing process in textile. Secondly, use of salt leads to maximum exhaustion of dye molecules during dyeing process in textiles.
Do you need to add salt to dye solution?
Contrary to some old wives' tales, salt is not a dye fixative and does nothing to make dye more permanent; however, it aids in the dyeing process by helping to drive the dye onto the fiber, out of solution, so that it is in the right place for any bonding to the fiber to occur.
What is the function of salt and soda in reactive dye?
Salts plays important role in reactive dyeing by improving the affinity of the dyestuff towards the fiber and acceleration of the dyestuff's association and lowering its solubility. Normally, Glauber's salt or common salt/ vacuum salt is used for this purpose.
How does salt work in dyeing cotton fiber with sulfur or reactive dye?
The salt in the reactive dyeing increases the affinity of the dye towards the Cellulosic substrate. Salt increases the exhaustion rate of reactive dyestuffs. As reactive dyestuffs have a lower affinity, more inorganic salt is required when using reactive dyestuffs in order to accelerate absorption.
What is salt free dyeing?
The salt free dyeing used to dye cotton. In conventional Method of dyeing of cotton with reactive dyes, alkali PH is should maintain in the dye bath. This method requires more electrolytes for exhaustion and alkali for fixation. In this paper the fibre modification technique based on polyacrylamide was discussed.
Does salt fix dye?
The vinegar and salt helps to fix the dye into the fibres of the fabric.
Does salt remove dye from clothes?
Yes, you can. Adding salt to your wash cycle helps remove stains and armpit discoloration, and keeps colors bright.
Should you add salt to Rit dye?
If you are dyeing with Rit All-Purpose Dye: To enhance the color: (1) add 1 cup of salt when dyeing fabrics containing cotton, rayon, ramie or linen; (2) add 1 cup of vinegar when dyeing fabrics containing nylon, silk or wool. Add 1 teaspoon of dish detergent to help promote level dyeing.
Do you need salt to tie dye?
Tie Dyeing (Other dyes will require salt, but it is best to use fiber reactive dyes, for tie-dyeing cellulose fibers such as cotton and rayon, because they are both easier to use and produce much more satisfactory results.)
Why do we need salt for dyeing?
Also know, why do you need salt for dyeing? Salt plays this crucial role of catalyst. Salt has an extremely high affin ity for water. Broadly speaking, Salt is necessary for three ways, firstly, to drive dye into textile during the dyeing process in textile. Secondly, use of salt leads to maximum exhaustion of dye molecules during dyeing process in ...
How to tie dye with salt water?
Also, how do you tie dye with salt? Use one cup per gallon of water, or follow whatever recipe you have already. Add the salt to the water separately from the dye - dissolve the dye first in water, before adding to the tub full of salt water - because salt makes the dye less likely to dissolve in water. Similarly, you may ask, do you have ...
How much salt to use in Rit All Purpose Dye?
If you are dyeing with Rit All-Purpose Dye: To enhance the color: (1) add one cup of salt when dyeing fabrics containing cotton, rayon, ramie or linen; (2) add one cup of vinegar when dyeing fabrics containing nylon, silk or wool.
What is the chemical that affects the final color of yarn?
A mordant is a chemical that becomes part of the molecular bond between the fiber and the dye. Primarily these are metal salts. The mordant also affects the final color of the dye. Alum and tin are considered neutral mordants, because the resulting color on yarn is pretty much that of the color of the dye bath.
Can you use salt in tie dye?
We do not normally use salt in mixing our dyes for use in tie-dy eing, because the high concentration of dyes in the tie-dye mixtures, as well as the close direct application of the dye to the fiber, makes salt unnecessary. It can also cause problems by making the dye less soluble.
Why is salt important in dyeing?
Salt plays a crucial medium because it has an extremely high water-attraction nature. In the textile industry, industrial salt is used in the processing of fabrics and materials, such as for dyeing cotton and other cellulose materials.
How does salt act in dyeing?
Usually, in three ways salt is acted during the dyeing process, first, the dye molecule will get transported into fibre molecule. Secondly, dispersing the dye molecules fully into the fibres. Thirdly, it is used as an electrolyte for the dyes to get fixed to the cellulose fibres.
What is anionic dye?
Anionic dyes, which produce negative charges, are mostly used in the dyeing process (eg: Reactive dyes, Direct dyes, Acid dyes) it must repel. It requires attraction to bind the dye molecules to the fabric surface. An exhausting agent used commonly is NaCl. It attracts Na+ and cl- ions (common ion effect) when dissolved in water.
Why is reactive dye exhaustion high?
The reactive dyestuff exhaustion is high during dyeing because of adding salt. Reactive dyes have a lower affinity towards cellulosic material. So, more inorganic salt to be used to accelerate the absorption while using reactive dyes. Generally, either Glauber’s salt or the common salt (sodium chloride) are used in dyeing.
What is the formula for Glauber's salt?
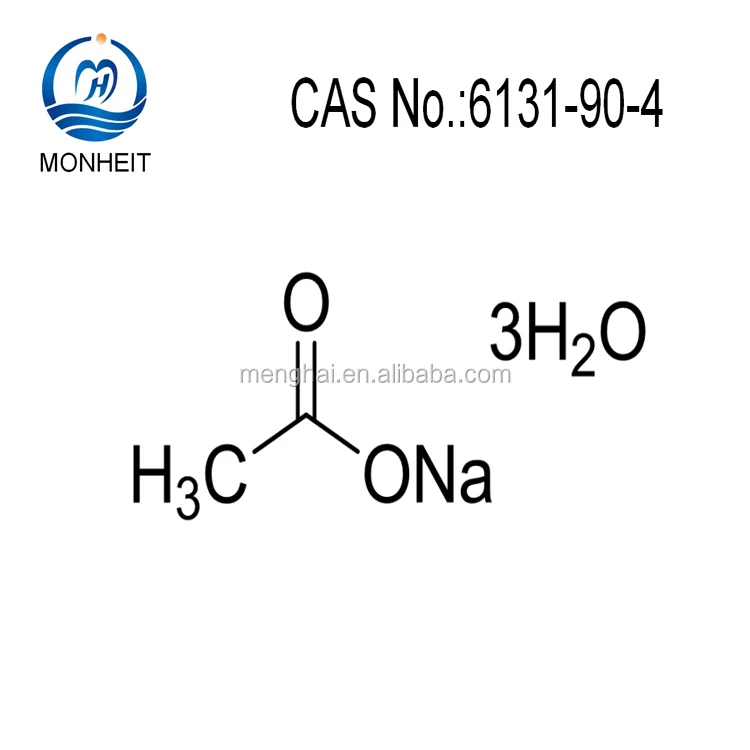
Glauber’s salt is the decahydrate form of sodium sulfate. The chemical formula of Glauber’s salt can be written as Na 2 SO 4. Common Salt is a chemical compound belonging to the larger class of salts composed primarily a mineral of sodium chloride (NaCl).
What is the exhausting agent used in dyeing?
An exhausting agent used during the dyeing process is normally a salt. The fabric will be immersed in water during dyeing which will generate negative charges on the fabric surface and this is called as Zeta potential. Anionic dyes, which produce negative charges, are mostly used in the dyeing process (eg: Reactive dyes, Direct dyes, ...
Is Glauber salt the same as sodium chloride?
Generally, either Glauber’s salt or the common salt (sodium chloride) are used in dyeing. Because of its efficiency and less cost. These two are essentially the same in terms of their place as an inorganic salt. Because of the presence of sodium cation in both the salts. image source.
Why is salt important in textiles?
Generally, Salt is critical in three ways, firstly, to drive dye into textile throughout the colouring method in textile. Secondly, use of salt results in most exhaustion of dye molecules throughout coloring method in textiles [4].
What is the method of concentration of salt in cotton coloring?
The method of the concentration of salt in cotton coloring was supported the principle of cationisation (to develop a positive charge) of cotton. The treated cotton once bleached from slightly acidic tub generates positive sites attributable to protonation within the amino.
Is reactive dyes good for clothes?
Reactive dyes square is measured very fashionable for clothes as they're environmentally safe and having sensible overall fastness properties. However application of those dyes needs an awfully high concentration of salt. The salt discharged from garment coloring is increased salinity in drain water stream that incorporates a negative impact on environmental ecology. The current work aims to eliminate the usage of salt throughout coloring of cotton merchandise with reactive dyes. The method of the concentration of salt in cotton coloring was supported the principle of cationisation (to develop a positive charge) of cotton. The treated cotton once bleached from slightly acidic tub generates positive sites attributable to protonation within the amino. The reactive dyes being anionic (negatively charged) in resolution get interested in the positive charges on the fiber that eliminates the salt needs for satisfactory dye exhaustion. After finished our work we found that Salt concentration for reactive dyes on textile materials is more important to show the good colorfastness, wash fastness, rubbing fastness.
Does salt increase the exhaustion of dye?
Salt increase the exhaustion and the attraction between the dye molecule and textile material [5]. Typically dye (direct & reactive) molecules have charges and therefore the material has also negative charge on its surface. Salt decreases the repulsion of negative-negative charges and thence improves exhaustion [6].
Is salt a chemical or physical necessity?
The physical-chemical necessity of the utilization of salt and soda in reactive coloring is standard to any or all technicians. The salt is Associate in nursing exhausting agent to push the color towards polysaccharide molecules and therefore the alkali (soda ash) is hydrolyzing/fixing agent for the reactive color.
Is plastic dye reactive?
In current follow, plastic fibers are preponderantly colored with reactive dyes within the presence of a substantial quantity of salt and glued underneath alkalescent conditions. However, dye fixation potency on plastic fibres is usually low (varying from 50%-90%).
