How to calculate neutralization reaction?
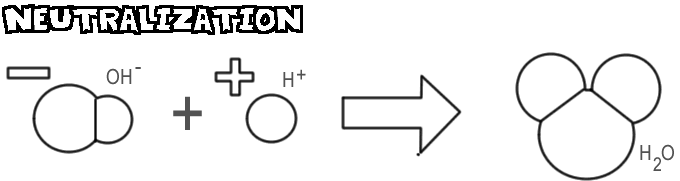
Nov 16, 2021 · When an acid and a base respond, the reaction is called a neutralizationreaction That’s because the reaction generates neutral items. Water is constantly one item, and a salt is additionally created. Favorable hydrogen ions from HCl and adverse hydroxide ions from NaOH incorporate to develop water. Is NaOH an acid or base?
What is the reaction between an acid and a base?
May 25, 2020 · When an acid and a base react, the reaction is called a neutralization reaction. That's because the reaction produces neutral products. Water is always one product, and a salt is also produced. Positive hydrogen ions from HCl and negative hydroxide ions from NaOH combine to form water. Click to see full answer.
What is the formula for neutralization reaction?
Feb 08, 2022 · Neutralization reaction is the process in which an acid reacts with a base to produce salt and water while evolving a good amount of heat. In this reaction, both the acid and base loose their properties to produce a new substance which is neutral in nature, i.e., the salt formed will neither be acidic nor basic.
What are some examples of a neutralization reaction?
A neutralization reaction is when a hydronium ion from an acid reacts with a hydroxide ion from a base to make water and a salt. According to Bronsted and Lowry, an acid-base reaction is simply when one molecule gives another molecule a proton. A conjugate base is a base that forms when an acid loses a proton.

Why are reactions between an acid and a base called neutralization?
When an acid and a base react, the reaction is called a neutralization reaction. That’s because the reaction produces neutral products. Water is always one product, and a salt is also produced. … Positive hydrogen ions from HCl and negative hydroxide ions from NaOH combine to form water.
Why is neutralisation called neutralisation?
H+ and OH- ions will readily combine to form water which is neutral. Note that both of the products formed (salt and water) are neutral. This is why the reaction is called a neutralisation reaction. The name of the salt produced in a neutralisation reaction can be derived from the acid and base used.
Is a neutralization reaction the same as an acid base reaction?
A neutralization reaction is when a hydronium ion from an acid reacts with a hydroxide ion from a base to make water and a salt. According to Bronsted and Lowry, an acid-base reaction is simply when one molecule gives another molecule a proton.
What do you call the chemical reaction between an acid and a base?
The reaction of an acid with a base is called a neutralization reaction. The products of this reaction are a salt and water.
What is the other name of neutralisation reaction?
The reaction in which an acid react base to give a salt and water is known as a neutralization reaction.
What type of reaction is neutralization reaction?
A neutralization is a type of double replacement reaction. A salt is the product of an acid-base reaction and is a much broader term then common table salt as shown in the first reaction.
What are the two products in every chemical reaction between acid and a base?
When an acid and a base are placed together, they react to neutralize the acid and base properties, producing a salt. The H (+) cation of the acid combines with the OH (-) anion of the base to form water. The compound formed by the cation of the base and the anion of the acid is called a salt.
What is neutralization reaction?
Continue Reading. In Neutralization, the transfer of a hydroxide ion (H+) from the acid to the base. They are therefore usually “displacement reactions,” but can also be combination reactions. The products are a salt and usually water. Therefore, they are also called “water-forming reactions.”. An example is when you take an antacid ...
What happens when you mix an acid and a base?
When u mix an acid with a base the reaction is a neutralization reaction. It is so called because the acid neutralizes the base. A neutralization reaction is when an acid and a base react to form salt and water and it involves the combination of H+ ions 0H- to form water.
What is the transfer of hydroxide ion (H+) from the acid to the base?
the transfer of a hydroxide ion (H+) from the acid to the base. They are therefore usually “displacement reactions,” but can also be combination reactions. The products are a salt and usually water. Therefore, they are also called “water-forming reactions.” An example is when you take an antacid to neutralize stomach acid from an upset stomach.
Acid and Base Difference
What is the primary acid and base difference? An acid is a chemical that is capable of acting as a proton donor. A proton is a hydrogen cation {eq}H^+ {/eq}. A base is a chemical that is capable of acting as a proton acceptor. The role that acids and bases play is complementary to one another.
Acid-Base Reactions
What is an acid-base reaction? The acid-base reaction definition describes the chemical change that occurs in a reaction between acid and base. The base reaction with a proton donor, an acid, leads to the exchange of protons and electrons between these two compounds. Acids donate their protons in exchange to the bases' electrons.
What is an example of a neutralization reaction?
What is one example of a neutralization reaction? A neutral ionic compound is a salt. Let’s see how both water and salt are created by a neutralisation reaction, using the reaction between hydrochloric acid solutions and sodium hydroxide as an example.
What is the best way to neutralize acidic soil?
For the neutralisation of acidic soils, farmers use lime (calcium oxide). There is hydrochloric acid in the gut, and so much of this induces indigestion. To neutralise the excess acid, antacid tablets contain bases such as magnesium hydroxide and magnesium carbonate.
What are the conditions for plant growth?
For optimal plant growth in any soil, there are certain conditions which are required. Some examples of materials mixed in the soil to neutralize it from acidity are: 1 Calcium Carbonate (Limestone) 2 Calcium Hydroxide (Slaked lime)
What is the purpose of nanomaterial synthesis?
3. Nanomaterial synthesis: To facilitate the chemical reduction of metal precursors, the heat of a neutralization reaction is used. 4. In our digestive systems: When food is moved between our stomach and intestines, the food needs to be neutralized.
What is a weak base?
A weak base is used to neutralise acids. Bases have a flavour that is bitter or astringent and have a pH greater than 7. Sodium hydroxide, potassium hydroxide and ammonium hydroxide are widely used.
Does toothpaste neutralize acid?
Toothpaste contains bases that neutralise the acid that our mouth creates from bacteria. To make the cakes grow, baking powder is typically used. To learn more about the different types of chemical reactions like decomposition reaction and more, register with BYJU’S.
What is neutralization reaction?
A neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products.
What is the reaction between an acid and a base called?
The reaction between an acid and a base invariably gives salt and water and is called neutralisation.
