Why is water a good recrystallization solvent? Because of its polarity and ability to form hydrogen bonds, water makes an excellent solvent, meaning that it can dissolve many different kinds of molecules. What is the most powerful solvent?
What is the difference between recrystallization and dissolving?
While most recrystallizations involve dissolving a solid solute in hot solvent, and cooling to release the solid from solution, there is another scenario. If the solid is unstable when heated, then we may dissolve it in a cold (room temperature) solvent, and then cool the solution to a lower temperature to precipitate out most of the solute,
What is the best solvent for recrystallization?
A second solvent, typically with a lower b.p., but the compound of interest is very soluble in it (e.g. diethyl ether), can be used in the recrystallisation.
What is the purpose of recrystallization?
ABSTRACT Recrystallization is the primary method for purifying solid organic compounds through the differences in solubility at different temperatures. In this experiment, a suitable solvent was first determined. Acetanilide was produced by acetylation of aniline with acetic anhydride.
How do you recrystallize acetanilide?
Choosing of the recrystallizing solvent was done by placing a small amount of pure acetanilide in three test tubes, each containing: water, 95% ethanol and hexane. The final weight produced by the pure acetanilide is 1.1g contrary to the weight of the crude of the acetanilide which is 0.5g.

Why is water used as a solvent in recrystallization?
A lot of times water is used for recrystallization of organic chemicals because they DON'T want to dissolve in such an extremely polar liquid (and it's so cool that water is so cheap!) but at 100 deg C, the temperature weakens the intermolecular attractions, forcing the organic to fall apart.
What makes a good solvent for recrystallization?
An ideal crystallization solvent should be unreactive, inexpensive, and have low toxicity. It is also important that the solvent have a relatively low boiling point (b.p. often <100oC as it's best if the solvent readily evaporates from the solid once recovered.
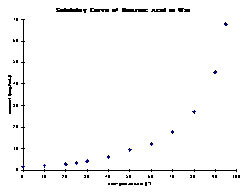
Why is water a good solvent for recrystallization of benzoic acid?
Data suggests that Water is the best solvent that will allow for better saturation and the best recrystallization of benzoic acid, this is largely due to water being a polar molecule whose properties allow for carboxylic acid groups, such as the one found in benzoic acid, to disassociate and donate protons to the water ...
Why is water and ethanol used for recrystallization?
Ethanol/water combinations are commonly used because ethanol has good dissolving ability for many organics, but is also infinitely co-soluble with water. Addition of water can rapidly and dramatically reduce the solubility of many organics and thus induce crystallization.
Which solvent are mostly used in crystallization?
waterThe most common solvent used for crystallization is water.
Is water or ethanol a better solvent for benzoic acid?
Addition of more ethanol in water favors the solubilization of benzoic acid.
Can water be used to recrystallize benzoic acid?
Benzoic Acid was recrystallized with a 41% recovery using 95% ethanol and water as the mixed-solvent. Benzoic acid was also recrystallized with a 79% recovery using water as the solvent. The product was a white crystalline solid (MP 114-122C and 121-127C respectively) after recrystallization.
Why is cold water used in recrystallization?
Typical problems: Crystallization can be a slow process, and impatience can lead to low recovery. The solution is placed in an ice-water bath to lower the temperature even further, and allow more crystals to form. At this point, most crystals should already have formed.
Why is ethanol a bad solvent for recrystallization?
As single solvent, ethanol is also a poor choice because the solubility of the compound is too low at high temperatures.
Why can a water ethanol solution be used for recrystallization but not a water hexane solution?
Answer and Explanation: Due to their same polarity, water and ethanol are miscible together forming a uniform solution. On the other hand, hexane, a non-polar solvent, cannot homogeneously mix a polar water.
Why does water dissolve benzoic acid?
The primary reason benzoic acid dissolves only slightly in cold water is that, even though the carboxylic acid group is polar, the bulk of the benzoic acid molecule is non-polar (water is polar). It is only the carboxylic group that is polar.
What is solvent required for the recrystallization of benzoic acid?
The initial purification will take place starting with impure benzoic acid, using water as a solvent to isolate pure benzoic acid. Once the benzoic has been re-crystallized (and purified away from NaCl), a percent yield and a melt temp analysis of the purified (and dried) product will be performed.
Does benzoic acid react with water?
Reasons for Poor Solubility in Cold Water The primary reason benzoic acid dissolves only slightly or poorly in cold water is that, because of a polar carboxylic group, the bulk amount of the benzoic acid molecule is non-polar. It is only the carboxylic group that is polar.
Is benzoic acid soluble in water?
Benzoic acid or benzene-carbonic-acid is a monobasic aromatic acid, moderately strong, white crystalline powder, very soluble in alcohol, ether, and benzene, but poorly soluble in water (0.3 g of benzoic acid in 100 g of water at 20 °C).
What is the primary method for purifying solid organic compounds through the differences in solubility at different temperatures?
ABSTRACT Recrystallization is the primary method for purifying solid organic compounds through the differences in solubility at different temperatures. In this experiment, a suitable solvent was first determined. Acetanilide was produced by acetylation of aniline with acetic anhydride. The crude acetanilide was dissolved in a solvent in a heating water bath. The hot solution was filtered and the ...
What is the best solvent for recrystallizing?
This experiment aims to purify crude acetanilide water , the best recrystallizing solvent, which is determined by the solubility of the solid compound, to produce pure acetanilide and to calculate the percentage yield of the weight of the pure acetanilide. Also, it aims to prove the process of recrystallization in purifying compounds.
How to purify organic solids?
Recrystallization is a technique used to purify organic solids. This method involves dissolving of a solute in a solvent and inciting the solute to produce a precipitate from a solution. In this experiment, acetic anhydride was added to the mixture of 2mL aniline and 20mL of distilled water. The mixture was cooled in an ice bath and filtered through filter paper resulting to the crude acetanilide. The pure acetanilide was then produced by the filtered solution of crude acetanilide and recrystallizing solvent. Choosing of the recrystallizing solvent was done by placing a small amount of pure acetanilide in three test tubes, each containing: water, 95% ethanol and hexane. The final weight produced by the pure acetanilide is 1.1g contrary to the weight of the crude of the acetanilide which is 0.5g. Quantitative analysis showed that the pure acetanilide produced 37.04% percentage yield.
How is crude acetanilide formed?
Crude acetanilide was formed from the synthesis of acetanilide. Pure acetanilide was collected after recrystallizing the crude acetanilide.
What is the process of purifying organic compounds that contain soluble, insoluble and colored impurities?
Recrystallization is a process of purifying organic compounds obtained from nature that contain soluble, insoluble and colored impurities. This is based on the premise that solubility increases on the increase of temperature while solubility decreases on the decrease of temperature.
What is the limiting reagent for acetic anhydride?
The limiting reagent is Aniline since 2.97g was needed to make 3 milliliters of Acetic Anhydride.
Why is crude oil a good solvent for recrystallization?
Because crude oil is a mixture of widely varying ... Choosing the best recrystallizing solvent would lead to the solubility of the impure substances in the compound. The boiling point of the recrystallizing solvent should be lower than the compound to be recrystallized.