first solvent of your choice. Note that water is a good solvent because it is non-toxic and non-flammable but that it does not evaporate readily. A sample recrystallized from water must be allowed to dry at least overnight. The solid will be filtered either by the pipet method or by suction filtration. If it seems that
What is meant by a good solvent for recrystallization?
What is meant by a good solvent for recrystallization? It means capable of dissolving and recrystallizing stuff. The normal means of purification of a compound are by recrystallization.
Why are both alcohols used in recrystallization?
Ideally, (which things never are!) a solute should be quite soluble in hot solvent, and poorly soluble in the cold solvent, so that on cooling the bulk of the solute crystallizes out. Both alcohols tend to fulfill these criteria with regard to recrystallization of many organic species.
Why are ethanol and methanol considered water like solvents?
Both ethanol and methanol are water like solvents (by virtue of the hydroxyl group); for this reason, and on the principle that like dissolves like, organic solutes should not have great solubilities in the cold solvent! Both alcohols are also cheap.
Why is water used as a recrystallization solvent?
A lot of times water is used for recrystallization of organic chemicals because they DON'T want to dissolve in such an extremely polar liquid (and it's so cool that water is so cheap!) but at 100 deg C, the temperature weakens the intermolecular attractions, forcing the organic to fall apart.
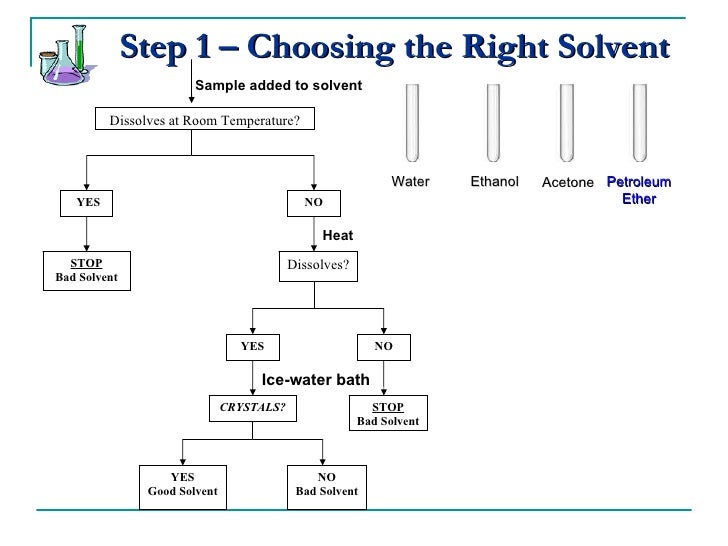
What makes a good solvent for recrystallization?
A good recrystallization solvent should (1) dissolve a moderate quantity of the substance being purified at an elevated temperature, but only a small quantity at low temperatures, (2) not react with the substance being purified, (3) dissolve impurities readily at a low temperature or not dissolve them at all, and (4) ...
Can water be a recrystallization solvent?
For most organic compounds, water is not a good recrystallization solvent. Recrystallization requires significant patience so be prepared to be patient. If you observe particles in the solution still, use a gravity filter remove them (hot gravity filtration).
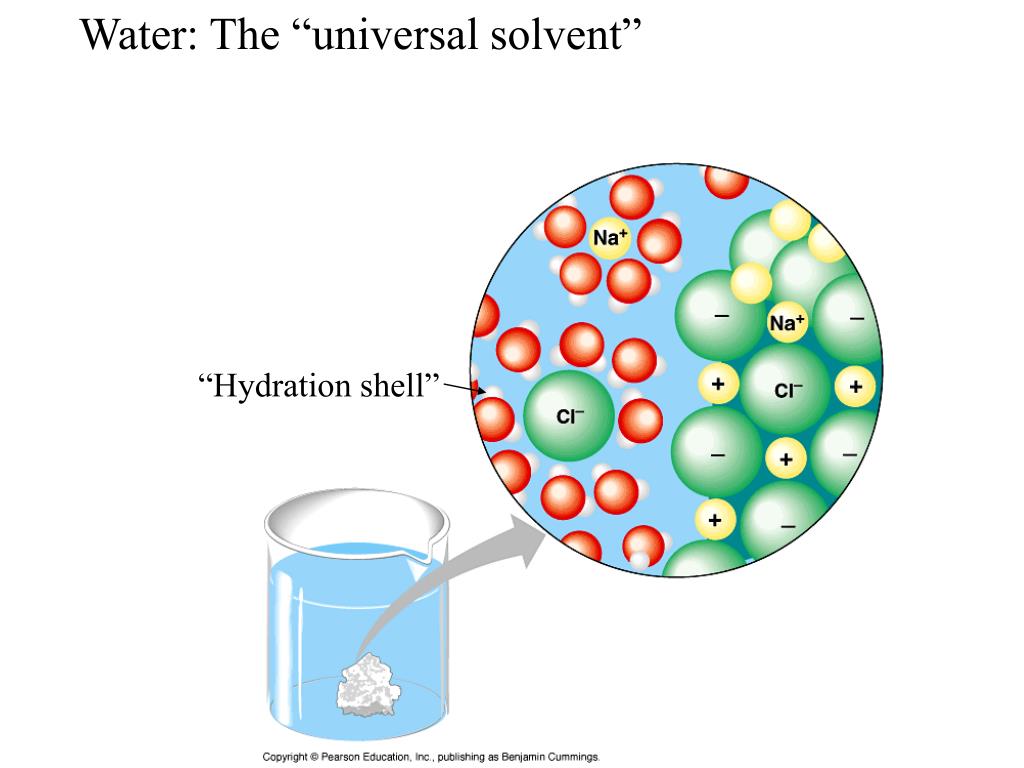
Why is water a good solvent for recrystallization of benzoic acid?
Data suggests that Water is the best solvent that will allow for better saturation and the best recrystallization of benzoic acid, this is largely due to water being a polar molecule whose properties allow for carboxylic acid groups, such as the one found in benzoic acid, to disassociate and donate protons to the water ...
Why is water and methanol used in recrystallization?
1 Answer. It means capable of dissolving and recrystallizing stuff.
Why is ethanol and water a good solvent for recrystallization?
Ethanol/water combinations are commonly used because ethanol has good dissolving ability for many organics, but is also infinitely co-soluble with water. Addition of water can rapidly and dramatically reduce the solubility of many organics and thus induce crystallization.
What advantages and disadvantages does water have as a recrystallization solvent?
Water. Answer: Advantage is its low cost and low toxicity; disadvantage is the difficulty of removing it from products due to low volatility.
Why can a water ethanol solution be used for recrystallization but not a water hexane solution?
Answer and Explanation: Due to their same polarity, water and ethanol are miscible together forming a uniform solution. On the other hand, hexane, a non-polar solvent, cannot... See full answer below.
Why is hot water used in recrystallization?
For the single-solvent and the two-solvents recrystallization method it is essential that you prepare a hot, saturated solution. To do this, all solvents must be hot before you add them. Heating the solvents decreases the kinetic energy necessary to dissolve the compound.
Why water is a good solvent for the recrystallization of Acetanilide?
The crude solid is dissolved in the smallest possible amount of solvent of choice; in this case the solvent is water. Acetanilide has a much higher solubility in hot water than in cold water.
Can benzoic acid be recrystallized in water?
Benzoic Acid was recrystallized with a 41% recovery using 95% ethanol and water as the mixed-solvent. Benzoic acid was also recrystallized with a 79% recovery using water as the solvent. The product was a white crystalline solid (MP 114-122C and 121-127C respectively) after recrystallization.
How does benzoic acid react with water?
0:001:25Benzoic Acid + Water = ?? (Weak Acid Ionization) - YouTubeYouTubeStart of suggested clipEnd of suggested clipBenzoic acid in water is not a real reaction at all benzoic acid is a weak acid. Which will giveMoreBenzoic acid in water is not a real reaction at all benzoic acid is a weak acid. Which will give away or donate an h to water.
What are the characteristics of a suitable solvent?
It is usually desirable if the solvent is non-toxic and not flammable. Unfortunately, few solvents are known to meet both criteria... Some solvents are not toxic but flammable (e.g., diethyl ether, hydrocarbons--petroleum ether, hexanes). Some are not flammable but toxic (e.g., dichloromethane, chloroform).
What solubility behavior describes a good solvent for your recrystallization?
You will simply be told to recrystallize a compound. A good recrystallization solvent is one in which the solid has a very high solubility at high temperatures and a very low solubility at low temperatures.
What makes a solvent pair too good?
A solvent which is "too good" will not allow recovery of much of the compound. On the other hand, if the solvent is "too poor," an excessively large volume of solvent would be needed. A solvent should be fairly volatile, because after the compound is collected, it must be freed of adsorbed solvent.