Is O2 an ionic or covalent bond?
So, Is O2 ionic or covalent? O2 is a covalent molecule because each oxygen atom needs two valence electrons to complete its octet. To meet this need, each oxygen atom shares two of its electrons with the other oxygen forming a strong oxygen-oxygen double shared covalent bond.
Is O2 considered a molecule or a compound?
So O2 is a compound. One single piece of O2 is a molecule. Oxygen occurs naturally as O2 so that is the elemental form of oxygen, so it's an element too. Oxygen does have another form, O3, ozone, but that isn't stable and gradually turns into O2 all by itself.
Are two oxygen molecules formed by a polar covalent bond?
Two covalent bonds form between the two oxygen atoms because oxygen requires two shared electrons to fill its outermost shell. … It gets these four from four hydrogen atoms, each atom providing one. These elements all share the electrons equally, creating four nonpolar covalent bonds. Why is oxygen bond polar?
Is O2 polar or nonpolar?
The oxygen (O2) molecule is nonpolar because the molecule is diatomic and both atoms have identical electronegativity. Both oxygen atoms share equal charges and there are no partial charges on any atom. Therefore O2 is a nonpolar molecule with a zero dipole moment.
Is O2 a polar covalent bond?
For starters, since the electrons would be evenly distributed between the two oxygen atoms, molecular oxygen (O2) is nonpolar. These materials similarly divide the electrons with the atoms of carbon and hydrogen, forming a non-polar covalent molecule. Was this answer helpful?
Is O2 polar covalent or ionic?
So, Is O2 ionic or covalent? O2 is a covalent molecule because each oxygen atom needs two valence electrons to complete its octet. To meet this need, each oxygen atom shares two of its electrons with the other oxygen forming a strong oxygen-oxygen double shared covalent bond.
Why is O2 polar covalent?
0:000:47Is O2 Polar or Non-polar? (Oxygen Gas) - YouTubeYouTubeStart of suggested clipEnd of suggested clipTo determine if o2 is a polar or nonpolar molecule we'll start with the lewis structure thatMoreTo determine if o2 is a polar or nonpolar molecule we'll start with the lewis structure that describes where those valence electrons are around the molecule. And it helps us determine polarity. So for
What type of bond is formed in O2?
covalent bondsA: The two oxygen atoms share two pairs of electrons, so two covalent bonds hold the oxygen molecule together.
What type of bond exist in O2?
double covalent bondHence, Oxygen form a divalent anion O2− and the type of bond present in oxygen molecule is a double covalent bond.
What bonds are polar covalent?
A polar covalent bond occurs when atoms are shared unequally in a covalent bond. Specifically, when the difference in electronegativities of the two atoms in the bond is between 0.4 and 1.7. The terms polar bond and polar covalent bond are generally used interchangeably.
Is co2 a polar covalent bond?
Carbon dioxide is symmetric and the pull of the two oxygens on the carbon's electrons cancel out, so it is a nonpolar molecule with polar bonds.
How do I know if a bond is polar?
Although there are no hard and fast rules, the general rule is if the difference in electronegativities is less than about 0.4, the bond is considered nonpolar; if the difference is greater than 0.4, the bond is considered polar.
How is O2 nonpolar?
Explanation : Diatomic oxygen is made up of the same two elements, and they equally share the 4 electrons that make up the double bond between them. They're equally electromagnetic, which means that there are not any partial charges for each element. Since neither atom pulls harder, it's a non- polar covalent bond.
Is co2 ionic or covalent?
covalentNo, CO2 is not an ionic compound. As per the definition, an ionic compound is a compound that is mostly formed between a metal atom and a non-metal atom. Meanwhile, CO2 is a compound that is formed between two non-metal atoms (carbon and oxygen) thus giving it a covalent nature.
Is O2 a double covalent bond?
Oxygen (O2) The oxygen molecule consists of two oxygen (O) atoms. Each oxygen has only six electrons and requires two more to complete its outermost shell. Therefore, the two electrons from each oxygen bond together. By sharing the four electrons, the oxygen molecule displays a double covalent bond.
What are the two types of covalent bonds?
Types of Covalent Bonds: Polar and Nonpolar. Electrons are shared differently in ionic and covalent bonds. Covalent bonds can be non-polar or polar and react to electrostatic charges. Ionic bonds, like those in table salt (NaCl), are due to electrostatic attractive forces between their positive (Na+) and negative charged (Cl-) ions.
Why are symmetrical molecules nonpolar?
Symmetrical molecules are nonpolar. Because nonpolar molecules share their charges evenly, they do not react to electrostatic charges like water does. Covalent molecules made of only one type of atom, like hydrogen gas (H2), are nonpolar because the hydrogen atoms share their electrons equally.
What is the ionic bond analogy?
a. Ionic bond analogy. The thief puppy has both bones (i.e. both electrons). The other puppy has lost its bone (electron). The puppies are held together because of the electrostatic force caused by their charge difference.
Why does hydrogen have a positive charge?
The hydrogen atom has a slightly positively charge because it cannot hold as tightly to the negative electron bones. Covalent molecules with this type of uneven charge distribution are polar. Molecules with polar covalent bonds have a positive and negative side. a. Ionic bond analogy.
Which molecule does not share electrons equally?
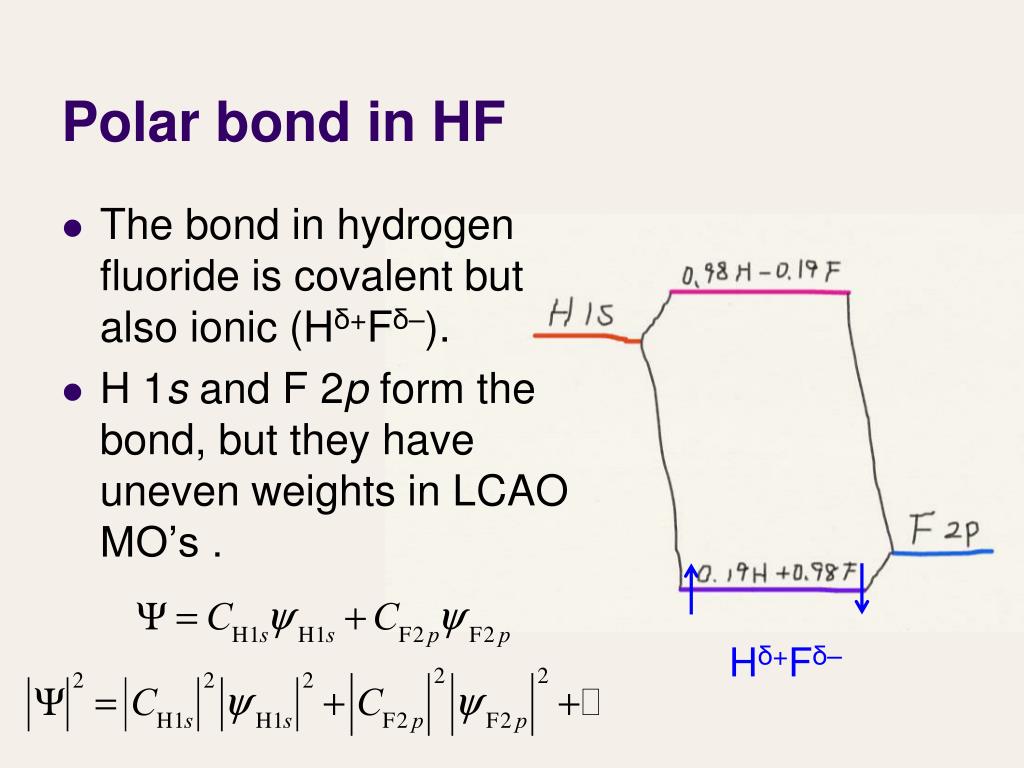
Other covalently bonded molecules, like hydrogen fluoride gas (HF), do not share electrons equally. The fluorine atom acts as a slightly stronger puppy that pulls a bit harder on the shared electrons (see Fig. 3-1c).
Which direction are water molecules normally oriented?
Fig. 3-5: Water molecules are normally randomly oriented (left) unless they are orienting themselves in their presence of an electrostatic force (right).
Is water a covalent molecule?
Water is a Polar Covalent Molecule. Water (H2O), like hydrogen fluoride (HF), is a polar covalent molecule. When you look at a diagram of water (see Fig. 3-2), you can see that the two hydrogen atoms are not evenly distributed around the oxygen atom. The unequal sharing of electrons between the atoms and the unsymmetrical shape ...
What type of bond is polar?
A polar bond is a type of covalent bond in which the electrons forming the bond are unequally distributed. In other words, the electrons spend more time on one side of the bond than the other.
Why do polar covalent bonds interact with dipoles?
Because positive and negative charges are separated in the bond, molecules with polar covalent bonds interact with dipoles in other molecules. This produces dipole-dipole intermolecular forces between the molecules. Polar bonds are the dividing line between pure covalent bonding and pure ionic bonding. Pure covalent bonds (nonpolar covalent bonds) ...
What type of bond is formed between two nonmetal atoms?
Polar covalent bonds form between two nonmetal atoms that have sufficiently different electronegativities from each other. Because the electronegativity values are slightly different, the bonding electron pair isn't equally shared between the atoms. For example, polar covalent bonds typically form between hydrogen and any other nonmetal.
What is a nonpolar covalent bond?
Pure covalent bonds (nonpolar covalent bonds) share electron pairs equally between atoms. Technically, nonpolar bonding only occurs when the atoms are identical to each other (e.g., H 2 gas), but chemists consider any bond between atoms with a difference in electronegativity less than 0.4 to be a nonpolar covalent bond.
What is polar bond?
A polar bond is a covalent bond between two atoms where the electrons forming the bond are unequally distributed. This causes the molecule to have a slight electrical dipole moment where one end is slightly positive and the other is slightly negative. The charge of the electric dipoles is less than a full unit charge, so they are considered partial charges and denoted by delta plus (δ+) and delta minus (δ-). Because positive and negative charges are separated in the bond, molecules with polar covalent bonds interact with dipoles in other molecules. This produces dipole-dipole intermolecular forces between the molecules.
How do ionic bonds form?
Ionic bonds form between atoms when the electronegativity difference between them is greater than 1.7. Technically ionic bonds are completely polar bonds, so the terminology can be confusing.
What are some examples of polar bonds?
Examples of molecules with polar bonds include water, hydrogen fluoride, sulfur dioxide, and ammonia.
What is the bond between oxygen and hydrogen?
Covelant bond is mutual sharing of electron between different atom. The bond between oxygen and hydrogen atom is covelant bond. Hydrogen and oxygen needed electrons to complete their valence electrons . So hydrogen and oxygen share their electrons and form a covalent bond.
What is the difference between ionic and polar bonds?
If there is zero difference in EN, the bond is called pure covalent. If there is a large difference in EN (let’s say greater than 2.0), the bond is said to be ionic . If the difference in EN is between these values (less than 2.0 but greater than zero), the bond is called polar covalent. The EN of Oxygen is about 3.5 and that of Hydrogen is about 2.2. This puts the difference in EN at about 1.3, which makes this a polar covalent bond. As
How to tell if a bond is ionic or covalent?
When determining the type of bond formed between two atoms, the most important consideration is the difference in electronegativity (EN) between the two atoms. If there is zero difference in EN, the bond is called pure covalent. If there is a large difference in EN (let’s say greater than 2.0), the bond is said to be ionic. If the difference in EN is between these values (less than 2.0 but greater than zero), the bond is called polar covalent. The EN of Oxygen is about 3.5 and that of Hydrogen is about 2.2. This puts the difference in EN at about 1.3, which makes this a polar covalent bond. As you continue your study chemistry, you will come to realize that it is misleading to categorize the type of bonding between two atoms as an absolute. It is better to think of bonding on a scale that ranges from “predominantly ionic” to “predominantly covalent” with degrees of ionic and covalent character in between.
What type of bond does hydrogen form?
Hydrogen reacts with oxygen to form a polar covalent bond. Explain why? - Quora
Why does hydrogen have an apolar covalent bond?
The hydrogen molecule has an apolar covalent bond H–H because the two hydrogen atoms that form the bond have obviously the same electronegativity: therefore , the electrons of molecular orbital that connects the two atoms is homogeneously distributed between the two hydrogen atoms. This kind of covalent bond is called “apolar”.
What happens when hydrogen reacts with oxygen?
When hydrogen react with oxygen will form two covalent bonds, but in this case the electronegativity of Oxygen and hydrogen are different.
Why does fluorine polarize?
Because fluorine IS electronegative, it polarizes electron-density towards itself to form a molecular dipole of the form…
What is a covalent bond?
A covalent chemical bond is one in which: a) Protons or neutrons are shared by two atoms so as to satisfy the requirements of both. b) Electrons are removed from one atom and transferred to another atom so that the two atoms become oppositely charged.
Is nitrogen electronegative?
a) Yes, because nitrogen is very electronegative.