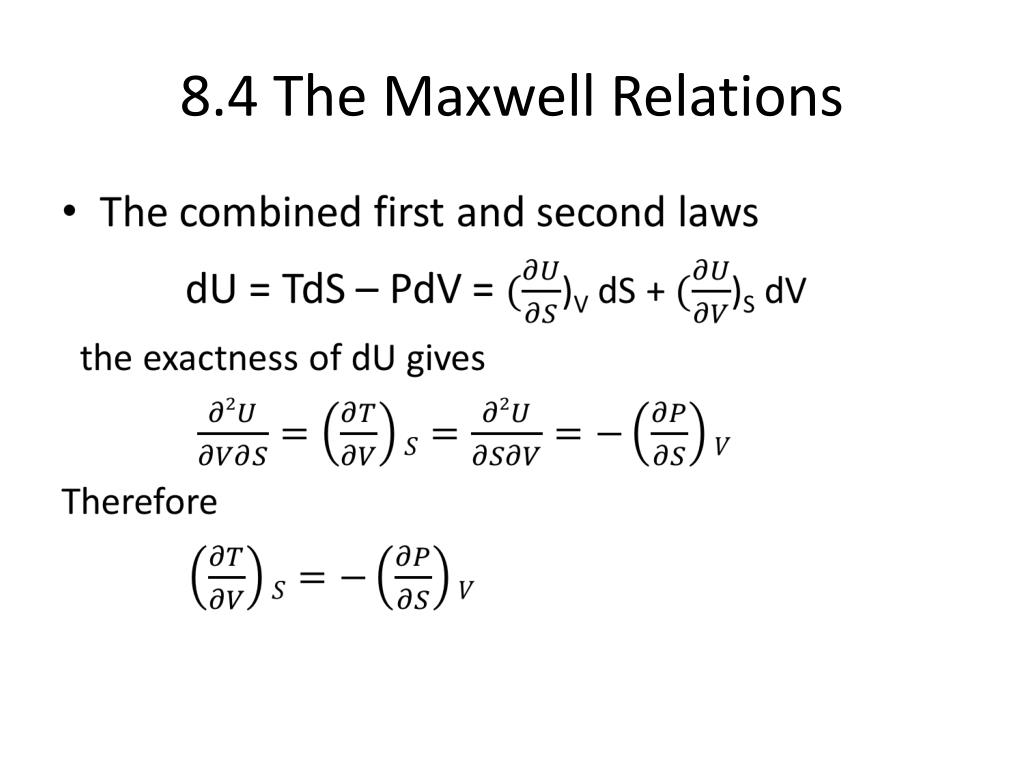
What is isochoric process?
Heat " In thermodynamics, an isochoric process, also called a constant-volume process, an isovolumetric process, or an isometric process, is a thermodynamic process during which the volume of the closed system undergoing such a process remains constant.
Are all isothermal and isobaric processes reversible?
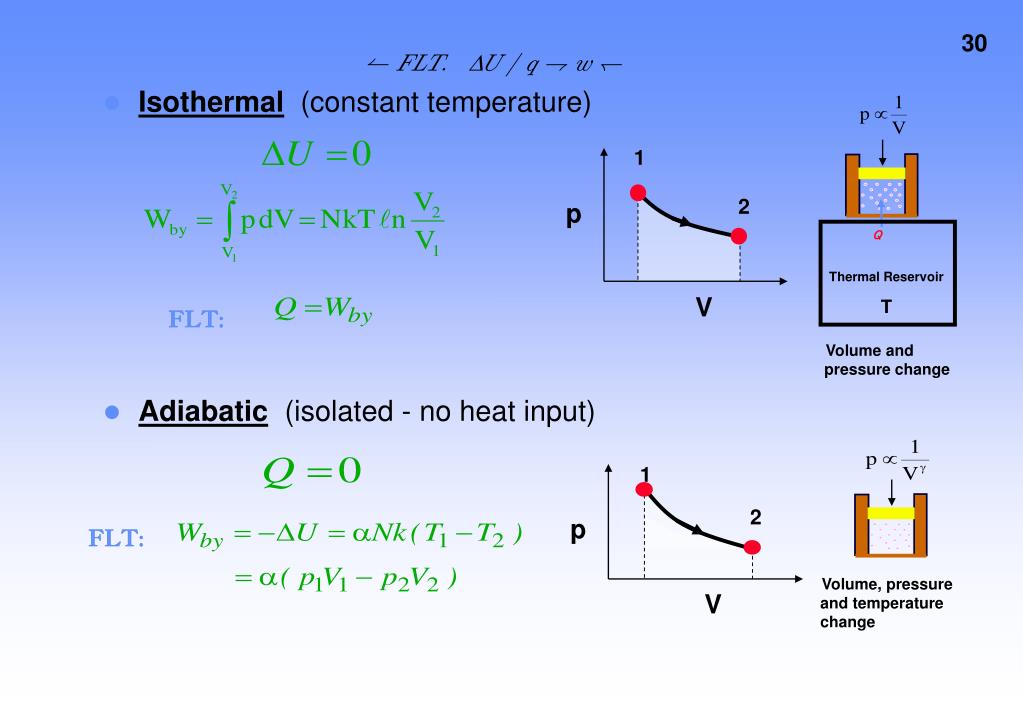
All isothermal and adiabatic process when allowed to proceed slowly, are reversible, provided there is no loss of energy against any type of resistance. Friction, viscosity etc. Examples of some reversible processes are given below. Below are the locus of isothermal, isobaric, isochoric and adiabatic process.
What happens to the volume of a gas during isochoric process?
Work Done by a Gas in an Isochoric Process In an isochoric process, the volume of the gas remains constant. For example, suppose that you have an ideal gas in a closed rigid container, heating the gas will raise its pressure without changing its volume. However, the quantity of gas remains constant.
What are the conditions for a process to be reversible?
For a process to be reversible the intensive macroscopic properties i.e. pressure and temperature, of the system and its surroundings must differ by, at most, an infinitesimal amount.

Are isobaric process reversible?
Isobaric processes are processes that occur under constant pressure. So if we are able to control the change in other macroscopic parameters of the system such that they change slow enough, yes you can carry out isobaric process slowly and reversibly.
Are all processes reversible?
In reality, almost all processes are irreversible, and some properties of the environment are altered when the properties of the system are restored.
What happens in an isochoric process?
In an isochoric process, the volume of the gas remains constant. For example, suppose that you have an ideal gas in a closed rigid container, heating the gas will raise its pressure without changing its volume. However, the quantity of gas remains constant.
Which of the following will not be a reversible process?
Solution(By Examveda Team) Since the entropy of mixing is positive we can say the mixing process or solution formation process is an irreversible process.
Which process is reversible?
Examples of Reversible Processes slow adiabatic compression or expansion of gases. electrolysis (with no resistance in the electrolyte) the frictionless motion of solids. slow isothermal compression or expansion of gases.
Why is an isochoric process irreversible?
There is no change in the heat and the work, and the changes in entropy and internal energy of the system comprised of the working fluid. The only change is that there is less of a decrease in the entropy of the surroundings in the irreversible path than for the reversible path.
Is isochoric process closed system?
In thermodynamics, an isochoric process, also called a constant-volume process, an isovolumetric process, or an isometric process, is a thermodynamic process during which the volume of the closed system undergoing such a process remains constant.
Which statement is true for isochoric process?
Correct option a In an isochoric process pressure remains constant. Explanation:In isochoric process it is volume that is kept constant. If pressure is kept constant it is an isobaric process.
What is the isochronic process?
An isochoric process, also called a constant-volume process, an isovolumetric process, or an isometric process, is a thermodynamic process during which the volume of the closed system undergoing such a process remains constant. An isochoric process is exemplified by the heating or the cooling of the contents of a sealed, inelastic container: The thermodynamic process is the addition or removal of heat; the isolation of the contents of the container establishes the closed system; and the inability of the container to deform imposes the constant-volume condition. The isochoric process here should be a quasi-static process .
Is the Otto cycle isochoric?
The ideal Otto cycle is an example of an isochoric process when it is assumed that the burning of the gasoline -air mixture in an internal combustion engine car is instantaneous. There is an increase in the temperature and the pressure of the gas inside the cylinder while the volume remains the same.
Problem 1
Tweleve grams of Nitrogen (N 2) in a steel tank are heated from 25.0 to 125ºC. (a) How many moles of nitrogen are present? (b) How much heat is transferred to the nitrogen?
Solution
Number of moles (n) is equal to given mass of molecule (m) divided by molecular weight (W) of the molecule,
Problem 2
A thermometer of mass 0.055 kg and heat capacity 46.1 J/K reads 15.0ºC. It is then completely immersed in 0.300 kg of water and it comes to the same final temperature as the water. If the thermometer reads 44.4 ºC, what was the temperature of the water before insertion of the thermometer, neglecting other heat losses?
Solution
In accordance to the law of conservation of energy, for a thermodynamic system, in which internal is the only type of energy the system may have, the law of conservation of energy may be expressed as,
Problem 3
An aluminum electric kettle of mass 0.560 kg contains a 2.40 kW heating element. It is filled with 0.640 L of water at 12.0ºC. How long will it take (a) for boiling to begin and (b) for the kettle to boil dry? (Assume that the temperature of the kettle does not exceed 100ºC at any time.)
Solution
The amount of heat per unit mass that must be transferred to produce a phase change is called the latent L for the process. The total heat transferred in a phase change is then
What is an example of an isochoric process?
Isochoric Process Example. A good example of an isochoric process is the ideal Otto cycle. In this, when the gasoline-air mixture is burnt in a car’s engine there is an increase in the temperature and the pressure of the gas inside the engine. Meanwhile, the volume of the gas remains exactly the same.
What is the work done by a gas in an isochoric process?
Work Done by a Gas in an Isochoric Process. In an isochoric process, the volume of the gas remains constant. For example, suppose that you have an ideal gas in a closed rigid container, heating the gas will raise its pressure without changing its volume. However, the quantity of gas remains constant.
What is isochoric process?
An isochoric process is a thermodynamic process, in which the volume of the closed system remains constant (V = const). It describes the behavior of gas inside the container, that cannot be deformed. Since the volume remains constant, the heat transfer into or out of the system does not the p∆V work, but only changes the internal energy (the temperature) of the system.
Is an isentropic process a thermodynamic process?
An isentropic process is a thermodynamic process, in which the entropy of the fluid or gas remains constant. It means the isentropic process is a special case of an adiabatic process in which there is no transfer of heat or matter. It is a reversible adiabatic process . The assumption of no heat transfer is very important, since we can use the adiabatic approximation only in very rapid processes.
Isobaric Process
For a thermodynamics process if the pressure remains constant for a system, then the process is called an isobaric process.
Isochoric Process
For a thermodynamics process, if the volume remains constant for a system, the process is known as an Isochoric process
Isothermal Process
According to Boyle’s law for a fixed mass of gas at a constant temperature, the volume is inversely proportional to the pressure. That means that, for example, if you double the pressure, you will halve the volume. This can express this mathematically as
Adiabatic Process
An adiabatic process is a thermodynamic process in which there is no exchange of heat between the system and surrounding, that is, the heat remains constant,
Reversible and Irreversible Process
A reversible process is one that is performed in such a way that at the final stage of the process both the system and surroundings return to their initial state with absolutely no change. In reality, there is no reversible process.