How to naturally increase fetal hemoglobin?
“Nettle is a herb that is a good source of B vitamins, iron, vitamin C and can play a key role in raising your hemoglobin level”, says Dr. Adarsh Kumar, Internal Medicine, National Heart Institute. All you need to do, is add 2 teaspoons of dried nettle leaves to a cup of hot water and allow it to steep for 10 minutes.
What increases fetal hemoglobin?
- Vaccinate against influenza, pneumonia, and hepatitis. ...
- Blood transfusions provide healthy red blood cells while slowing the productions of ones that will never work properly. ...
- The drug hydroxyurea increases fetal-hemoglobin levels, among other benefits.
- Drugs called chelators can clear excess iron from the body. ...
What are normal HCT and HGB levels?
Normal levels of hematocrit for men range from 41% to 50%. Normal level for women is 36% to 48%. Hemoglobin Hemoglobin enables red cells to transport oxygen and carbon dioxide throughout your body. Normal hemoglobin for men ranges from 13.5 to 17.5 g/dL. Normal range for women is 12.0 to 15.5 g/dL. Hemoglobin is checked before each blood donation.
When does fetal to adult hemoglobin switch over occur?
The switch to produce adult forms of hemoglobin (essentially hemoglobin A) starts at around 40 weeks of gestation, which is close to the expected time of birth. At birth, hemoglobin F accounts for 50-95% of the infant's hemoglobin and at around 6 months after birth, hemoglobin A becomes the predominant type.

Does everyone have fetal hemoglobin?
While common in fetuses, in normal adults, only around 3-7% of red blood cells contain hemoglobin F.
Which age is fetal hemoglobin still present in the body?
In individuals without hemoglobinopathies, it is almost completely replaced by adult hemoglobin (hemoglobin A, HbA, alpha2beta2) by approximately 6 to 12 months of age, and it amounts to less than 1 percent of total hemoglobin in adulthood.
Can adults have HbF?
Healthy adults usually only have HbF in tiny amounts. If your amount is higher, you may have a blood disorder, such as thalassemia or sickle cell anemia. Or you may have another condition related to high levels of HbF.
How much fetal hemoglobin do adults have?
Abstract. The synthesis of fetal hemoglobin (HbF) is normally reduced to very low levels of less than 0.6% of the total hemoglobin in adults. The HbF is restricted to a sub-population of erythrocytes termed 'F-cells'; 85% of the normal adult population have 0.3% to 4.4% F-cells.
Can fetal hemoglobin persist throughout life?
Hereditary persistence of fetal hemoglobin (HPFH) is a benign condition in which significant fetal hemoglobin production continues well into adulthood, disregarding the normal shutoff point after which only adult-type hemoglobin should be produced.
At what age does HbF change?
From ∼85% of total hemoglobin at birth, HbF normally falls to <1% at 1 year of age, with a reciprocal increase in adult hemoglobin (HbA). The switch from HbF to HbS in sickle cell anemia (homozygosity for the HbS gene) is delayed, and stable levels of HbF are not reached until age 5 to 10 years.
What will happen if HbF persist in adult life?
The persistence of HbF into adult life could be a non-pathogenic condition as in HPFH or be associated with other diseases states. HPFH is a rare benign asymptomatic inherited disorder with persistence of HbF into adult life [1].
What type of hemoglobin is in adults?
Normal adult hemoglobin contains 95% to 98% hemoglobin A (two alpha chains and two beta chains). It also contains 2% to 3% Hb A2 (normal variant with two alpha chains and two delta chains) and 1% to 2% fetal hemoglobin consisting of two alpha and two gamma chains.
What type of hemoglobin do adults have?
Many different types of hemoglobin (Hb) exist. The most common ones are HbA, HbA2, HbE, HbF, HbS, HbC, HbH, and HbM. Healthy adults only have significant levels of only HbA and HbA2. Some people may also have small amounts of HbF.
What is the difference between HbF and HbA?
Blood transfusion with adult haemoglobin (HbA) replaces foetal haemoglobin (HbF). HbA has a lower affinity for oxygen than HbF and therefore leads to increased oxygen availability to the tissues including the retina.
Why is fetal Haemoglobin different to adult haemoglobin?
Fetal hemoglobin binds to oxygen more strongly than adult hemoglobin, enabling the transfer of oxygen from mother to fetus prenatally. Oxygen exchange within the tissue is thus affected by the strength of the binding between hemoglobin and oxygen.
When did HbF change to HbA?
In healthy humans, a shift from γ-globin to β-globin gene expression around birth underlies the switch from fetal (α2γ2; HbF) to adult (α2β2; HbA) hemoglobin production, such that by 6 months of age the major hemoglobin is HbA.
What is the lifespan of fetal RBC?
The mean life span of all red cells in the fetal circulation was 63.6 +/- 5.8 days.
Why HbF is replaced by HbA?
Blood transfusion with adult haemoglobin (HbA) replaces foetal haemoglobin (HbF). HbA has a lower affinity for oxygen than HbF and therefore leads to increased oxygen availability to the tissues including the retina.
Why is fetal hemoglobin shift left?
The leftward shift in the ODC ensures an oxygen uptake at a lower partial oxygen pressure (pO2) for fetus in utero, as well as a lower oxygen extraction at capillary beds in peripheral tissues (1–5) (Figure 1).
What is the BCL11A protein?
BCL11A protein levels appeared to correlate with the developmental stage of expression, such that primitive and fetal liver definitive erythroid cells that expressed high levels of γ-globin, had low or absent expression of the full-length forms of BCL11A.
What is hemoglobin made of?
The hemoglobin molecule is a tetramer composed of two subunits of α-like globin peptide chains and two subunits of the β-like globin peptides, along with heme moieties necessary for this molecule’s oxygen-carrying capacity. A variety of β-like globin molecules are produced as a result of the fact that the human β-globin locus on chromosome 11 is developmentally regulated (Fig. 1) (Sankaran and Nathan 2010a; Sankaran et al. 2010). In the early part of the first trimester, there is robust expression of an embryonic form of a β-like globin known as ε-globin within the yolk sac-derived primitive lineage of erythrocytes (Peschle et al. 1985). Soon thereafter, when production of the first enucleated definitive erythrocytes commences from stem and progenitor cells in the fetal liver (McGrath and Palis 2008), the predominant β-like globin molecule produced is γ-globin (Sankaran et al. 2010). This molecule is encoded by two duplicated γ-globingenes found within the β-globin gene cluster (Fig. 1). The γ-globin chains combine with adult α-globin chains into a stable tetramer forming HbF. This remains the predominant hemoglobin for much of gestation. Shortly after the time of birth there is a switch from predominant expression of HbF to adult hemoglobin (HbA), which is mediated by a transcriptional switch in definitive erythroid progenitors from γ-to β-globin(Fig. 1).
How are HBF regulators found?
Although the regulators of HbF regulation discussed above have been found through human genetic studies, thus confirming their in vivo relevance in humans, a variety of other molecules have also been suggested to play roles in γ-globingene regulation through various studies using cell culture approaches or with mouse models (Table 1). These molecules will be discussed below. It is important to bear in mind that there are limitations for most of the currently available experimental systems used to study γ-globingene regulation. Primary human erythroid cells seem to be permissive to manipulation that will allow increases in γ-globin production, which may not always be relevant in humans in vivo. In addition, many of the stimuli that are known to induce γ-globin production in vivo in humans, including various types of stress erythropoiesis and treatment with hydroxyurea, do not function to induce γ-globin production in humanized mouse models (Pace et al. 1994; Sankaran et al. 2009) stressing an important limitation for interpreting negative findings in this experimental system. Thus, caution needs to be exercised when interpreting experimental findings not supported by evidence from human genetic studies or studies performed in humans or primates in vivo.
What happens when you have a single base substitution?
In SCD, a single base substitution causes a missense mutation of a valine for glutamic acid at amino acid six of the β-globin protein chain, which leads to a propensity of the sickle hemoglobin to polymerize. This in turn results in deformation of erythrocytes containing this hemoglobin, which can block small blood vessels, leading to impaired oxygen delivery to tissues. This can result in significant clinical complications including pain crises, respiratory complications, and organ damage. In β-thalassemia, insufficient production of the β-globin molecule results in an excess of unpaired α-globin chains that can precipitate within erythroid precursors. The precipitation of these free α-globin chains impairs the maturation and leads to death of these precursors, causing ineffective production of erythroid cells. As a result, a significant anemia occurs and the consequent expansion of erythroid precursors can lead to secondary problems in bones and other organs.
Which receptors bind to the direct repeats of the -globingenes?
2011). Paradoxically, overexpression of TR2 and TR4 in transgenic mouse models results in elevated expression of γ-globin and when overexpressed in sickle cell disease mouse models can result in partial amelioration of hematologic and pathologic symptoms of these mice (Campbell et al. 2011). The mechanisms underlying these observations and the relevance to human globin gene regulation remains to be established for TR2 and TR4. In addition, the orphan nuclear hormone nuclear receptor COUP-TFII has also been suggested to bind to the direct repeats and repress the γ-globinpromoter in humans (Filipe et al. 1999). Using in vitro cultures of primary adult erythroid cells, it was shown that cytokines such as SCF appear to down-regulate expression and chromatin occupancy of COUP-TFII at the β-globin locus, resulting in increased γ-globin expression (Aerbajinai et al. 2009). Moreover, direct down-regulation of COUP-TFII using small interfering RNAs (siRNAs) could result in increases in γ-globin production (Aerbajinai et al. 2009). Further studies on the role of COUP-TFs in the regulation of HbF will be needed to better understand its physiologic role in humans.
What is the purpose of the fetal to adult hemoglobin switch?
The fetal-to-adult hemoglobin switch and silencing of fetal hemoglobin (HbF) have been areas of long-standing interest among hematologists, given the fact that clinical induction of HbF production holds tremendous promise to ameliorate the clinical symptoms of sickle cell disease (SCD) and β-thalassemia. In this article, we discuss historic attempts to induce HbF that have resulted in some therapeutic approaches to manage SCD and β-thalassemia. We then go on to discuss how more recent molecular studies that have identified regulators, including BCL11A, MYB, and KLF1, hold great promise to develop targeted and more effective approaches for HbF induction. We go on to discuss strategies by which such approaches may be developed. Older studies in this field can provide important lessons for future studies aimed at developing more effective strategies for HbF induction, and we therefore chronologically cover the work accomplished as this field has evolved over the course of the past four decades.
What happens if you have hemoglobin in your blood?
This in turn results in deformation of erythrocytes containing this hemoglobin, which can block small blood vessels, leading to impaired oxygen delivery to tissues. This can result in significant clinical complications including pain crises, respiratory complications, and organ damage.
How to determine fetal hemoglobin?
Fetal hemoglobin is useful in evaluating various conditions in pregnancy and the neonate. The hemoglobin alkaline denaturation test (Apt test) can help to differentiate maternal and fetal blood. In this test, a blood sample of 0.1 mL is added to a glass tube with an alkali reagent containing potassium hydroxide, and the solution is shaken gently for 2 minutes. HbA will bind hydroxide to form hematin, turning the sample a dark-green brown, which indicates the presence of maternal blood in the sample. If only fetal blood is present, the solution will remain pink. The Kleihauer-Betke test assesses the extent of maternal-fetal hemorrhage and the required dose of RhoD immunoglobulin for Rh-negative mothers, for the prevention of maternal Rh antibody formation leading to Rhesus disease in the fetus/neonate. The test utilizes HbF's property of acid resistance. A blood smear taken from the mother is exposed to an acidic pH solution; maternal red blood cells become "ghost cells," as HbA is unstable at a low pH, and the cell membrane is denatured. Since fetal blood cells contain HbF, they remain pink, as HbF is stable at this pH range. The appropriate dose of RhoD immunoglobulin is calculated based on the percentage of fetal blood cells in the maternal blood. [8][9][10]
How does hemoglobin evolve?
The evolution of hemoglobin follows gene mutations, such as gene duplication, gene conversion, and translocation of genes in ancient hemoproteins. Mutations that resulted in changes to the primary structure of globin altered its properties and genetic regulatory regions. The gamma-globin genes of fetal hemoglobin are products derived from duplications of beta-globin gene clusters. In the fetus, HbF is preceded by the embryonic hemoglobins, whose production in the yolk sac (weeks 3 through 8) decreases shortly after HbF is produced in the liver (weeks 6 through 30), followed by the spleen (9 through 28), and finally the bone marrow (28 through birth). Approximately all HbF is replaced by HbA by 6 to 12 months of age unless hemoglobinopathy is present in the individual; the average adult has less than 1% of HbF as a result. The switch from gamma to beta chain occurs through a transcriptional switch in erythroid precursor cells in the bone marrow. [7]
What is the role of fetal hemoglobin in the transport of oxygen?
Cellular. Fetal hemoglobin has a vital role in the transport of oxygen from maternal to fetal circulation. Oxygen transfer from the maternal circulation to the fetal circulation is made possible by HbF having a high oxygen affinity but decreased affinity to 2,3-bisphosphoglycerate relative to HbA.
What is the dominant form of hemoglobin present in the fetus during gestation?
Physiology, Fetal Hemoglobin - StatPearls - NCBI Bookshelf. Fetal hemoglobin (HbF) is the dominant form of hemoglobin present in the fetus during gestation. HbF is produced by erythroid precursor cells from 10 to 12 weeks of pregnancy through the first six months of postnatal life. HbF contains two alpha and two gamma subunits, ...
Why are red blood cells pink?
Since fetal blood cells contain HbF, they remain pink, as HbF is stable at this pH range.
What percentage of HBF is in sickle cell?
At baseline, HbF accounts for 2% to 20% of hemoglobin in sickle cell disease, depending on various patient-dependent factors, and this elevation appears to be due to the greater oxygen affinity of HbF; therefore, HbF is less likely to deoxygenate, sickle, and cause pain crises in these patients.
How long does it take for a hematocrit to produce HbF?
HbF is produced by erythroid precursor cells from 10 to 12 weeks of pregnancy through the first six months of postnatal life. HbF contains two alpha and two gamma subunits, while the major form of adult hemoglobin, hemoglobin A (HbA), contains two alpha and two beta subunits.
How do HBS differ from each other?
Different Hbs are found in humans during the embryonic, fetal and adult life and they differ from each other in the type of globin chain they possess. Except in the first few weeks of embryo formation, one globin chain pair is always alpha while the second pair is termed non-alpha - (beta , gamma , delta).
What is the protein that makes up hemoglobin?
A: Haemoglobin (Hb), the oxygen carrying protein present in our red blood cells, is made up of an iron containing Haeme molecule surrounded by a protein called Globin. Each Hb molecule contains two pairs of globin chains.
Why does the developing fetus not breathe?
The developing fetus does not breathe the way we do by drawing air into its lungs (where there is plenty of oxygen). Instead, it draws its oxygen from the mother's blood (where the oxygen content is low).
How does hemoglobin affect the growth of a baby?
Presence of high levels of hemoglobin F in pregnant women can impact the growth of the fetus, as fetal red blood cells struggle to compete for the oxygen from the mother's circulation . This is because instead of competing with hemoglobin A, which has a weaker association to oxygen than hemoglobin F, it becomes a competition between fetal and maternal hemoglobin F which have similar affinities for oxygen. As a result, women with hemoglobin F as >70% of total hemoglobin are much more likely to have fetuses that are small for their gestational age compared women with <70% hemoglobin F (at a rate of 100% compared to 8%, respectively).
Why are F cells higher in inherited disorders?
Due to the correlation between the amount of hemoglobin F and F-cells, F-cell numbers are higher in some inherited hemoglobin disorders, including beta-thalassemia, sickle cell anemia and hereditary persistence of fetal hemoglobin . Additionally, some acquired conditions can also have higher F-cell numbers, such as acute erythropoietic stress (response to poor oxygenation which includes very rapid synthesis of new red blood cells) and pregnancy. F-cells have similar mass of haemoglobin per cell compared to red blood cells without haemoglobin F, which is measured mean cell haemoglobin values (MCH).
What is the main form of hemoglobin in the fetus?
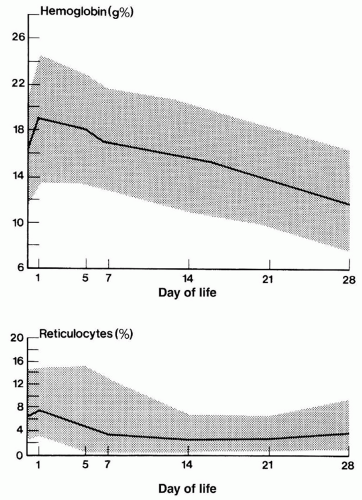
During the first 3 months of pregnancy, the main form of hemoglobin in the embryo/fetus is embryonic hemoglobin, which has 3 variants depending on the types of subunits it contains. The production of hemoglobin F starts from week 6, but it's only from 3 months onwards that it becomes the main type found in fetal red blood cells. The switch to produce adult forms of hemoglobin (essentially hemoglobin A) starts at around 40 weeks of gestation, which is close to the expected time of birth. At birth, hemoglobin F accounts for 50-95% of the infant's hemoglobin and at around 6 months after birth, hemoglobin A becomes the predominant type. By the time the baby is one year old, the proportions of different types of hemoglobin are expected to approximate the adult levels, with hemoglobin F reduced to very low levels. The small proportion of red blood cells containing hemoglobin F are called F-cells, which also contain other types of hemoglobin.
Why does hemoglobin F affect the fetus?
Firstly, the presence of hemoglobin F in the fet us allows a stronger binding to oxygen than maternal hemoglobin (see Factors affecting oxygen affinity ). Secondly, the mother's bloodstream is richer in oxygen that the fetus’, so oxygen naturally flows towards the fetal circulation by diffusion.
What is the main oxygen carrier protein in the fetus?
Fetal hemoglobin, or foetal haemo globin (also hemoglobin F, HbF, or α2γ2) is the main oxygen carrier protein in the human fetus. Hemoglobin F is found in fetal red blood cells, and is involved in transporting oxygen from the mother's bloodstream to organs and tissues in the fetus. It is produced at around 6 weeks of pregnancy and ...
How many subunits does hemoglobin F have?
Structure and genetics. Hemoglobin F, like adult hemoglobin ( hemoglobin A and hemoglobin A2 ), has four subunits or chains. Each subunit contains a heme group with an iron element which is key in allowing the binding and unbinding of oxygen.
Why is hemoglobin F higher than normal?
Diseases such as beta thalassemias, which affect components of the adult hemoglobin, can delay this process, and cause hemoglobin F levels to be higher than normal. In sickle cell anemia, increasing the production of hemoglobin F has been used as a treatment to relieve some of the symptoms.
Why do fetus have fetal hemoglobin ?
This fetal hemoglobin is a necessity of fetus, otherwise it can't get oxygen. Lungs of a fetus don't work inside mother's womb, and it can only absorb oxygen from mother's blood which is available till placenta.
At what time production of fetal hemoglobin begins ?
In fetus production of fetal hemoglobin begins in 6th week, by 3 months it becomes the main type of hemoglobin found in fetus. Around completion of 40th week, which is around the time of birth, production of adult hemoglobin begins. By the age of 6 months after birth, adult hemoglobin becomes the predominant type.
Why adults don't have fetal hemoglobin ?
If an adult female have fetal hemoglobin, then oxygen won't pass onto fetus, as both mother and fetus will have fetal hemoglobin and similar force to absorb oxygen. In such case fetus will die due to oxygen deficiency.
Is Fetal Hemoglobin a marker for Cancer ?
Yes it is ! Some researcher have found that in cancer patients, fetal hemoglobin is generally found in newly formed blood vessels of cancerous tumors. It makes the cancerous tumor grow easily by extracting more oxygen from blood.
How did hemoglobin evolve?
Evolution — Hemoglobin evolved from ancient hemoproteins by gene duplication, gene conversion (non-reciprocal exchange of genetic material between two linked homologous genes), translocation to different chromosomes, and mutations that caused changes in the primary structure and properties of globin and their various genetic regulatory regions [ 1 ].
What is the fetal hemoglobin?
Fetal hemoglobin (hemoglobin F, HbF, alpha2gamma2) is the major hemoglobin present during gestation; it constitutes approximately 60 to 80 percent of total hemoglobin in the full-term newborn. In individuals without hemoglobinopathies, it is almost completely replaced by adult hemoglobin (hemoglobin A, HbA, alpha2beta2) by approximately 6 to 12 months of age, and it amounts to less than 1 percent of total hemoglobin in adulthood.
Where does the breakpoint of a large deletion causing hereditary persistence of fetal hemoglobin occur?
The breakpoint of a large deletion causing hereditary persistence of fetal hemoglobin occurs within an erythroid DNA domain remote from the beta-globin gene cluster. Blood 1989; 74:2178.
What is Adekile's anthropological correlate?
Adekile AD. Historical and anthropological correlates of beta S haplotypes and alpha- and beta-thalassemia alleles in the Arabian Peninsula. Hemoglobin 1997; 21:281.
Is HBF a clinically relevant factor?
As a minor hemoglobin following the first year of life, HbF has little in the way of clinical relevance in normal physiology. However, it is assuming ever greater importance in certain of the hemoglobinopathies, in which congenital, acquired, and drug-induced increases in HbF have been shown to improve the clinical features of affected individuals with sickle cell disease and beta thalassemia. This important subject is discussed in depth separately. (See "Hydroxyurea use in sickle cell disease" .)
Is UpToDate a substitute for medical advice?
The content on the UpToDate website is not intended nor recommended as a substitute for medical advice, diagnosis, or treatment. Always seek the advice of your own physician or other qualified health care professional regarding any medical questions or conditions. The use of UpToDate content is governed by the UpToDate Terms of Use. ©2021 UpToDate, Inc. All rights reserved.
What is the percentage of fetal haemoglobin?
This is often termed hereditary persistence of fetal haemoglobin or HPFH for short. This can range from 1 to 100% Hb F for different people. Graph showing how haemoglobin gradually changes from the fetal form to the adult form. In most individuals (solid lines) the fetal form makes up less than 1% of the total haemoglobin ...
How much of the total haemoglobin is in the fetal form?
In most individuals (solid lines) the fetal form makes up less than 1% of the total haemoglobin by the time the individual is one year old. Individuals with HPFH (dashed lines) maintain higher levels of the fetal form. Hb F is a lot like Hb A. Adults who have no Hb A but can make enough Hb F are:
Why do women in scenarios 3 and 4 need follow up?
The women in scenarios 3 and 4 require follow-up because a small but significant minority could have a type of haemoglobin mutation known as delta beta thalassaemia. This does not affect the health of the woman assuming that she has no other mutations present in this region of her haemoglobinopathy genes.
What is a PHE blog?
PHE Screening blogs provide up to date news from all NHS screening programmes. You can register to receive updates direct to your inbox, so there’s no need to keep checking for new blogs. If you have any questions about this blog article, or about population screening in England, please contact the PHE screening helpdesk.
What happens when a sickle cell is deoxygenated?
In an individual with sickle cell disease, the red blood cell becomes misshapen and rigid, resembling the shape of a sickle, when the haemoglobin is de-oxygenated (releases the oxygen to the organs). This process is called ‘sickling’ and causes a wide range ...
What is the substance within red blood cells that carries oxygen around the body?
Haemoglobin (Hb) is the substance within red blood cells that carries oxygen around the body.
What happens if the father is not available for DNA testing?
If the father is not available for testing, the mother can be offered DNA testing to help establish if she has a mutation that might put the child at increased risk.
