What does C represent in chemistry?
Carbon (from Latin: carbo "coal") is a chemical element with symbol C and atomic number 6. It is nonmetallic and tetravalent—making four electronsavailable to form covalent chemical bonds.
What does concentration measure in chemistry?
concentration: The relative amount of solute in a solution. In chemistry, concentration of a solution is often measured in molarity (M), which is the number of moles of solute per liter of solution. This molar concentration (c i) is calculated by dividing the moles of solute (n i ) by the total volume (V) of the:
What does stoichiometric mean in chemistry?
stoichiometric ( ˌstɔɪkɪəˈmɛtrɪk) , stoicheiometric or stoechiometric adj 1. (Chemistry) concerned with, involving, or having the exact proportions for a particular chemical reaction: a stoichiometric mixture. 2. (Chemistry) (of a compound) having its component elements present in the exact proportions indicated by its formula 3.
What is CO2 in chemistry?
What is carbon dioxide?
- Carbon dioxide is a compound. Its molecules consist of one carbon atom joined to two oxygen atoms.
- In the atmosphere, carbon dioxide is a greenhouse gas.
- We use carbon dioxide to make drinks fizzy and keep foods fresh.
See more
Are CO and CO the same in chemistry?
CO and Co is completely different things the CO means for the carbon monoxide where as the Co is the single element named as Cobalt.
What does CO stand for chemistry?
Carbon MonoxideCarbon Monoxide (CO) is a colorless, odorless, tasteless, toxic gas that has the molecular formula CO. The molecule consists of a carbon atom that is triply bonded to an oxygen atom. Carbon monoxide is a commercially important chemical.
What is the difference between the representations CO and CO?
So, the${\text{CO}}$ is a molecule made up of carbon and oxygen atoms. Whereas the ${\text{Co}}$ represents an element named as Cobalt.
Is CO and CO2 same?
The major difference between Carbon Dioxide (CO2) and Carbon Monoxide (CO) is in the number of oxygen atoms they carry though both contain carbon and oxygen atoms. While both have the same number of carbon atoms, i.e., one carbon atom, carbon dioxide has 2 carbon atoms while carbon monoxide has one oxygen atom.
What is CO short for?
Co. is used as an abbreviation for company when it is part of the name of an organization. [business] ...the Blue Star Amusement Co.
What do CO and CO refer to?
The key difference between CO and Co is that CO is an inorganic compound consisting of carbon and oxygen atoms, whereas Co is the metal named Cobalt. CO and Co are two different chemical substances. CO is a colourless, odourless, and tasteless, flammable gas consisting of a carbon atom and an oxygen atom per molecule.
What would be wrong if we write CO for Cobalt instead of CO?
Answer: If we write CO it means it consists of two non-metals namely Carbon and Oxygen and it would represent Carbon monoxide but not Cobalt.
What type of molecule is CO?
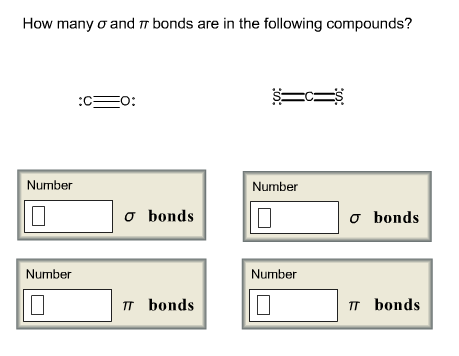
Carbon monoxide is a colorless, odorless, tasteless gas. A molecule of carbon monoxide (CO) contains one carbon atom and one oxygen atom. This image shows four representations chemists use for carbon monoxide.
How do you draw CO?
0:012:32How to Draw the Lewis Dot Diagram for Carbon monoxide (CO) - YouTubeYouTubeStart of suggested clipEnd of suggested clipWe'll start by putting the carbon here. And then the oxygen. So we have 10 total valence electronsMoreWe'll start by putting the carbon here. And then the oxygen. So we have 10 total valence electrons let's put 2 between atoms that'll form our chemical bond.
Does CO turn into CO2?
Carbon monoxide has a typical "lifespan" of several months in Earth's atmosphere. The gas eventually reacts with oxygen (O2) to form carbon dioxide (CO2).
Do cars emit CO2 or CO?
For an internal combustion engine to move a vehicle down the road, it must convert the energy stored in the fuel into mechanical energy to drive the wheels. This process produces carbon dioxide (CO2).
Is CO worse than CO2?
Carbon monoxide is a far more dangerous gas. Also referred to as the “Silent Killer,” carbon monoxide is a colorless, odorless, tasteless, and non-irritating gas, so the early signs of poisoning are difficult to detect.
What is the name of CO in chemistry?
Carbon monoxide (chemical formula CO) is a colorless, highly poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond.
Is CO ionic or covalent?
0:151:15Is CO (Carbon monoxide) Ionic or Covalent/Molecular? - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo answer to our question carbon monoxide it's covalent the electrons between the carbon and theMoreSo answer to our question carbon monoxide it's covalent the electrons between the carbon and the oxygen these are shared so that each atom has a full outer shell has an octet.
What is the name for the symbol CO?
cobalt (Co), chemical element, ferromagnetic metal of Group 9 (VIIIb) of the periodic table, used especially for heat-resistant and magnetic alloys.
Is CO a compound or element?
Carbon monoxideCarbon monoxide / IUPAC ID
What is CO made of?
CO is a compound that is made up of two different non-metal elements; carbon (C) and oxygen (O). Notice both letters are capital letters in the symbol, that is a clue that it consists of two different elements. Atoms of these two elements are covalently bonded to each other to form molecules that exist as a gas under standard conditions.
What is the symbol for cobalt?
Co is the chemical symbol for the metal element cobalt. It consists of only one type of atom - cobalt atoms. This element could not be given the symbol C because that symbol was already being used by carbon. So, for cobalt, they used the first letter of its name (in capital) as well as the second letter of its name (in lowercase) as its symbol.
Is the o capital in Cobalt?
in the element Cobalt, Co, the o is NOT a capital.
Is copernicium the same as cyanide?
No. They are chemically unrelated. Similarly, the cyanide group and copernicium are spelled with the same letters, as are praseodymium and the standard abbreviation for the propyl group. Coincidences in spelling are not that interesting usually - cobalt and CO are perhaps slightly more fun because of dicobalt octacarbonyl.
What is the difference between CO2 and CO2?
The biggest difference is that CO2 is a common , naturally occurring gas found everyday from decaying plant and animal life as well as geothermic activity. CO is not common. It is a byproduct of the burning of fossil fuels such as oil, coal, and gas. The media often adds to the confusion because their inability to discern the two gases adds to ...
What is the molecular weight of CO2?
Both are in the air worldwide (albeit in different concentrations) Both are released during combustion or fire. Both are potentially deadly. The molecular weight of CO is 28.01, where the molecular weight of CO2 is 44.1.
How much CO2 is dangerous?
Drowsiness can occur at 10,000 ppm (1%) – common in closed cars or auditoriums. Symptoms of mild CO2 poisoning include headaches and dizziness at concentrations less than 30,000 ppm (3%) At 40,000 ppm (4%) CO2 can be life-threatening.
What is the highest CO emissions?
The highest CO emissions are produced at dangerous levels by internal combustion engines. CO can be a flammable gas in higher concentrations (sometimes referred to as C1D1 or C2D2 environments) and devices to measure carbon monoxide in these concentrations are normally designed to be explosion-proof.
Why is the media so confused?
The media often adds to the confusion because their inability to discern the two gases adds to the issue. Countless stories abound about injuries or fatalities from CO poisoning when a gas fired generator is run inside a dwelling during natural disasters like hurricanes.
What are the symptoms of CO poisoning?
Symptoms of mild CO poisoning include headaches, dizziness, and violent vomiting at concentrations less than 100ppm
Where does CO2 collect?
CO2 will collect near floor level while CO will collect closer to the ceiling.
How does CO bond?
CO also has all its valence orbitals satisfied, but in a less satisfactory way, with a triple bond between the atoms. Basically we are asking C and O to both act like N, and O has one two many electrons and C one too few, so they have to work it out. O only has two unpaired electrons for bonding, so it puts a lone pair in the third bond- a dative bond. Carbon can thus only use two of its four unpaired electrons in the bonds, the other two go together to make a lone pair. Since O is donating both electrons in the dative bond, it ends up relatively positive while the C ends up relatively negative. Or you can think of taking N2, which is triple-bonded, nonpolar, and very stable; and removing a proton from one N to make C and adding it to the other to make O. Even after the electrons rearrange the O (N with the extra proton) will be more positive than the C (N missing a proton). And that lone pair on negative carbon apparently makes it a good coordinate covalent ligand for certain metal cations including Fe+2. (that is an oversimplification, Wikipedia has a clear explanation at Carbon monoxide - Wikipedia, but it is a bit over my head) Anyway this tendency to ligate Fe++ causes it to bind tightly to the heme iron in hemoglobin and myoglobin, much more tightly than nonpolar O2 despite steric advantages of the latter. It thus displaces O2 and drastically reduces the O2 carrying capacity of the blood. It also binds to heme a3 in cytochrome oxidase and various other pentacoordinate hemes, inhibiting their function.
What is the cause of CO2?
Carbon dioxide, CO2, is caused by the complete burning of any substance containing carbon. By "complete burning," I mean that the carbon atom becomes linked to two atoms of oxygen, which is a stable formation. Carbon dioxide is somewhat toxic to animals, including humans, which is why all animals expel if from their bodies when they breathe out. It is a little known fact that when people suffocate in a closed space, it is actually the carbon dioxide poisoning that kills them, not the lack of oxygen. That is, they die from CO2 poisoning well before all the available oxygen is used up. Carbon dioxide is also is the most important greenhouse gas, meaning that it traps the heat energy from sunlight in the air and causes the atmosphere to warm more than it normally would as infrared light passes through it. Carbon dioxide is not he most powerful greenhouse gas, but there is now so much more of it in the air from humans burning carbon than the other gases that its greenhouse contribution is more than any other gases.
Why is carbon monoxide toxic?
That means that it has latched onto only one atom of carbon and the burning process is not complete. Because of this, it is unstable and reactive, which is why it is so much more toxic than CO2. It latches onto the red blood cells and prevents them from capturing oxygen, causing death. Carbon monoxide is rare in the air, except near a source of incomplete burning, otherwise we would all be dead. Incomplete burning is usually caused by burning carbon inside a closed space where their is not enough oxygen to allow complete burning, such as inside a car engine. Carbon monoxide is also a greenhouse gas, but because it is so rare, it does not contribute much to global warming.
What is the cause of carbon dioxide?
Carbon dioxide, CO2, is caused by the complete burning of any substance containing carbon. By "complete burning," I mean that the carbon atom becomes linked to two atoms of oxygen, which is a stable formation. Carbon dioxide is somewhat toxic to animals, including humans, which is why all animals expel if from their bodies when they breathe out. It is a little known fact that when people suffocate in a closed space, it is actually the carbon dioxide poisoning that kills them, not the lack of oxygen. That is, they die from CO2 poisoning well before all the available oxygen is used up. Carbon di
What is a substance that inhibits another substance or a reaction?
3. Chemistry A substance that inhibits another substance or a reaction: a catalyst poison.
Is carbon monoxide dangerous?
Carbon monoxide is very dangerous and rather low levels can kill you or cause permanent organ and brain damage.
Is carbon dioxide the same as water?
Carbon monoxide (CO) and carbon dioxide (CO2) are about as different as water (H2O) and pure hydrogen peroxide (H2O2) and as different as oxygen (O2) and ozone (O3) and as different as benzene (C6H6) and Benzaldehyde, which has a benzene ring (C6H5CHO).