What is the element number 1 on the periodic table?
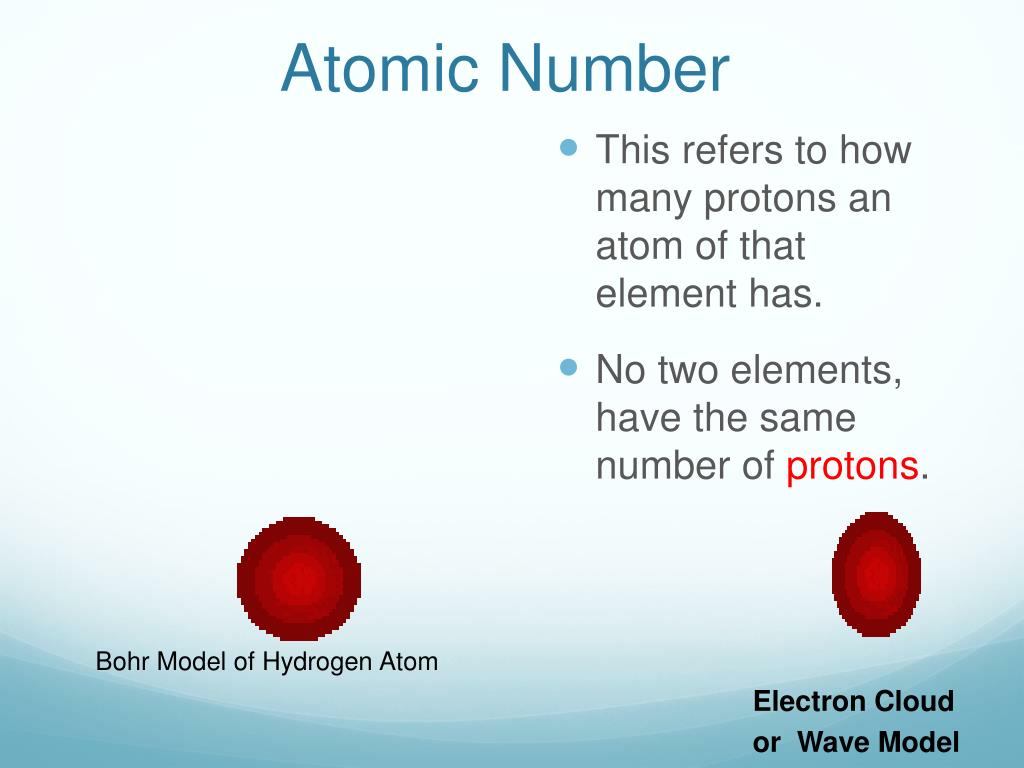
She has taught science courses at the high school, college, and graduate levels. Hydrogen is the element that is atomic number 1 on the periodic table. The element number or atomic number is the number of protons present in the atom. Each hydrogen atom has one proton, which means it has a +1 effective nuclear charge.
What is the element number of a hydrogen atom?
The element number or atomic number is the number of protons present in the atom. Each hydrogen atom has one proton, which means it has a +1 effective nuclear charge. At room temperature and pressure, hydrogen is a colorless, odorless gas.
How many isotopes of an atom have atomic number 1?
There are three isotopes that all have atomic number 1. While an atom of each isotope has 1 proton, they have different numbers of neutrons. The three isotopes are proton, deuterium, and tritium.
What is the oxidation number of 1 in an atom?
Many elements can exhibit a variety of oxidation states. While atomic number 1 usually displays a +1 oxidation state, it can also pick up a second electron and exhibit a -1 oxidation state. Because two electrons fill the s subshell, this is a stable configuration.
What does an atomic number of 1 mean?
The atomic number is equal to the number of protons in an atom's nucleus. Hydrogen's atomic number is 1 because all hydrogen atoms contain exactly one proton.
What has an atomic mass of 1?
ProtonProton has a mass of 1 and an atomic number of 1.
What element has an atomic number of 1 and mass number of 1?
hydrogenFor hydrogen, the atomic mass is 1 because there is one proton and no neutrons.
What is the name of 1 atom?
2.13). For example, hydrogen (H) has an atomic number of one (1). This means a hydrogen atom has one proton. If a hydrogen atom is neutral, it must also have one electron.
Do protons have an atomic mass of 1?
This is a tiny, dense region at the center of the atom. Protons have a positive electrical charge of one (+1) and a mass of 1 atomic mass unit (amu), which is about 1.67×10−27 kilograms.
What isotope has an atomic number of 1?
HydrogenHydrogen is a case in point. It has the atomic number 1.
Is the simplest element and has the atomic number of 1?
HydrogenHydrogen: a chemical element with atomic number 1 and symbol H. It is a colorless, odorless, nonmetallic, tasteless, highly flammable diatomic gas with the molecular formula H2. It has an atomic mass of 1.00794. Hydrogen is the most abundant element in the universe and it has an atomic number of 1 .
Which element has number of protons 1?
hydrogenFor example, hydrogen has an atomic number of 1. This means that an atom of hydrogen has one proton, and, if it's neutral, one electron as well.
Is an atom 1 element?
An atom is the part of an element. A particular element is composed of only one type of atom. Atoms are further composed of subatomic particles called electrons, protons and neutrons. Elements can combine with each other to form molecules via chemical reaction.
What is 1 proton called?
Protons are made up of fundamental particles called quarks (two up quarks and a down quark) held together by gluons. The nucleus of the simplest atom, Hydrogen (H), is a single proton.
What is a +1 ion called?
The loss of one or more electrons results in more protons than electrons and an overall positively charged ion, called a cation. For example, a sodium atom with one less electron is a cation, Na+, with a +1 charge (Figure 3.1. 1).
What has A mass of 1.0 amu?
Since protons and neutrons have a mass of 1.0 amu/particle, the atomic mass is the sum of the number of protons and neutrons or the number of neutrons is equal to the atomic mass minus the atomic number.
Which two particles each have A mass of 1?
Answer and Explanation: The two subatomic particles that have the same mass are protons and neutrons. Neutrons and protons each have an atomic mass of about one atomic mass unit (amu).