Melting points and boiling points of alkanes Boiling points increase with increasing chain length because of the increasing strength of the intermolecular forces. This allows the alkane mixture in crude oil to be separated by fractional distillation.
How can we separate an alkane mixture in crude oil?
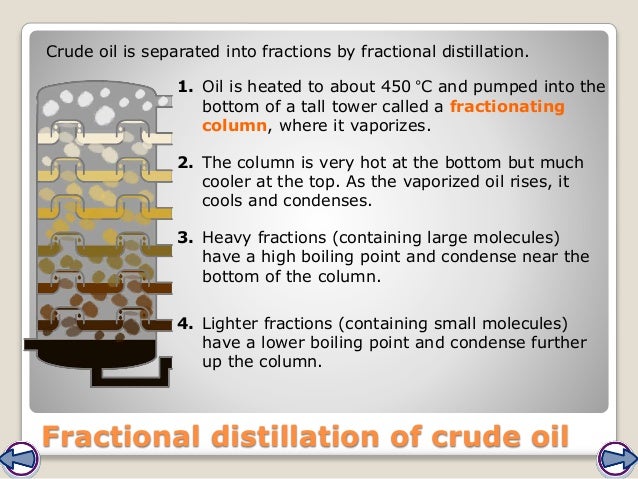
Feb 13, 2022 · How can a mixture of alkanes be separated? Fractional distillation heated crude oil enters a tall fractionating column , which is hot at the bottom and gets cooler towards the top. vapours from the oil rise through the column. vapours condense when they become cool enough. liquids are led out of the column at different heights.
How is crude oil separated into useful fractions by fractional distillation?
Feb 21, 2020 · Crude oil is heated to vaporize the different hydrocarbons in a tank which is cool at the top and hot at the bottom. The vapours then rise and the different hydrocarbons condense at their specific boiling points, allowing them to be separated. Click to see full answer. Moreover, how is the mixture of alkanes separated by fractional distillation? Fractional distillation …
How is petrol made from fractional distillation?
Crude oil is a finite resource. Petrol and other fuels are produced from it using fractional distillation. Cracking is used to convert long alkanes into shorter, more useful hydrocarbons.
What is the difference between cracking and fractional distillation?
Feb 22, 2011 · "Fractional" distillation simply means that you are using some condensing medium (i.e. steel wool) inside the distillation column. In either case, alkanes are a good compound because they are ...
How does fractional distillation separate alkanes?
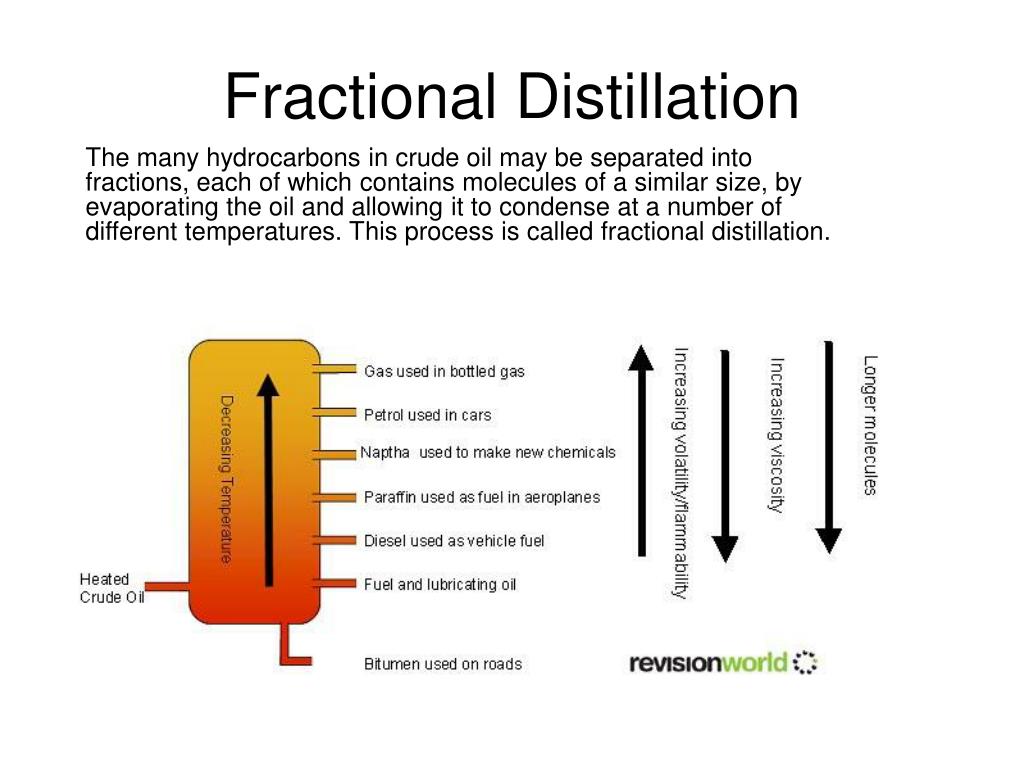
How does fractional distillation work? A common pattern in chemistry is that larger molecules have higher boiling points than smaller ones. Alkanes are no exception: the longer the chain, the higher the boiling point. Fractional distillation uses this fact to separate the chains.
How does fractional distillation separate the fractions in crude oil?
Crude oil is separated into fractions by fractional distillation. The fractions at the top of the fractionating column have lower boiling points than the fractions at the bottom. All of the fractions are processed further in other refining units.
What process separates crude oil into fractions?
Fractional distillationFractional distillation is the process by which oil refineries separate crude oil into different, more useful hydrocarbon products based on their relative molecular weights in a distillation tower.Sep 27, 2021
How does separated occur in fractional distillation?
In a fractional distillation, a mixture of liquids is boiled and the resulting vapors travel up a glass tube called a “fractionating column” and separate. The fractionating column is placed between the flask containing the mixture and the “Y” adaptor and improves the separation between the liquids being distilled.
Why does fractional distillation work with crude oil?
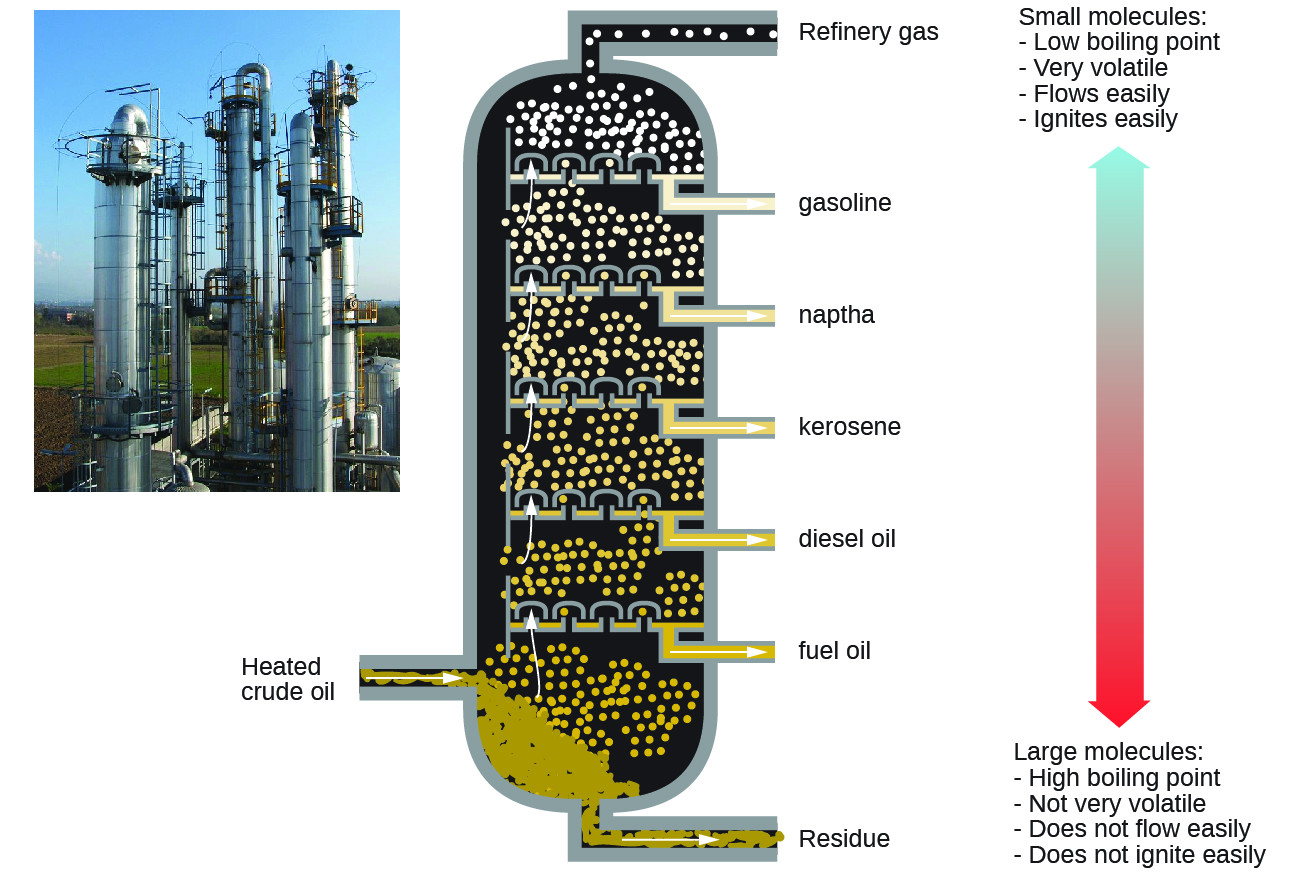
Crude oil is a mixture of hydrocarbons . The crude oil is evaporated and its vapours condense at different temperatures in the fractionating column. Each fraction contains hydrocarbon molecules with a similar number of carbon atoms and a similar range of boiling points.
How is crude oil refined by fractional distillation?
Petrol, Diesel and Kerosene are all products (or fractions) of the process of refining Petroleum. On an industrial scale, the different fractions of Petroleum are separated out by fractional distillation. The Petroleum is heated up, and the gas produced enters the fractionating column.Aug 19, 2020
How is crude oil separated into fractions by fractional distillation GCSE?
Fractional distillation heated crude oil enters a tall fractionating column , which is hot at the bottom and gets cooler towards the top. vapours from the oil rise through the column. vapours condense when they become cool enough. liquids are led out of the column at different heights.
What are the three stages in separating hydrocarbons from crude oil?
Three major types of operation are performed to refine the oil into finished products: separation, conversion and treating.Jan 6, 2015
How is crude oil processed to separate the different hydrocarbons for various uses?
Crude oil is made up of a mixture of hydrocarbons, and the distillation process aims to separate this crude oil into broad categories of its component hydrocarbons, or "fractions." Crude oil is first heated and then put into a distillation column, also known as a still, where different products boil off and are ...Jul 5, 2012
What type of mixture is separated by fractional distillation?
Fractional distillation is a method for separating a liquid from a mixture of two or more liquids. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. This method works because the liquids in the mixture have different boiling points.
How is distillation separated from oil and water?
Distillation Methods The first uses a high-boiling-point water-immiscible liquid such as a mineral oil. The sample is suspended in this oil and heated to a temperature high enough to allow the water to be vaporized. As the water is distilled off, it is condensed and collected in a vessel and measured.
What is the basis of the separation of different hydrocarbons in crude oil or petroleum?
fractional distillationThe main process for separating the hydrocarbon components of crude oil is fractional distillation. Crude oil fractions separated by distillation are passed on for subsequent processing into numerous products, ranging from gasoline and diesel fuel to heating oil to asphalt.
Where do the gases leave in the crude oil column?
The diagram below summarises the main fractions from crude oil and their uses, and the trends in properties. Note that the gases leave at the top of the column, the liquids condense in the middle and the solids stay at the bottom.
What is crude oil?
Crude oil is a mixture of hydrocarbons. The crude oil is evaporated and its vapours condense at different temperatures in the fractionating column. Each fraction contains hydrocarbon molecules with a similar number ...
Does coal cause acid rain?
Coal is mainly carbon but it may also contain sulfur compounds, which produce sulfur dioxide when the coal is burned. This gas is a cause of acid rain. Also, as all fossil fuels contain carbon, the burning of any fossil fuel will contribute to global warming due to the production of carbon dioxide. previous.