
How are silicates formed?
These types of silicates are also known as Phyllosilicates. These are formed by sharing three bridging oxygen per silicon atom. So a two-dimensional sheet is formed.
What are the key concepts of silicate minerals?
Key Concepts. Silicate minerals are the most common of Earth's minerals and include quartz, feldspar, mica, amphibole, pyroxene, and olivine. Silica tetrahedra, made up of silicon and oxygen, form chains, sheets, and frameworks, and bond with other cations to form silicate minerals.
What are some examples of minerals made of silica?
These include minerals such as quartz, feldspar, mica, amphibole, pyroxene, olivine, and a great variety of clay minerals. The building block of all of these minerals is the silica tetrahedron, a combination of four oxygen atoms and one silicon atom.
Where are silicate minerals formed?
Some silicates form deep beneath Earth's surface. As molten magma begins to harden, crystals slowly form. Other silicates can form in the spaces between rocks. As superheated liquids flow through cracks, they grab particles from the rocks around them, which then precipitate into mineral veins.
How is silica formed naturally?
It is formed when silicon is exposed to oxygen. It has a covalent bond and is a superior electric insulator, posessing high chemical stability. Quartz is the second most common mineral in the Earth's continental crust.
How is silica rock formed?
It occurs in beds and in nodules. Bedded chert consists of siliceous fossils such as diatoms and radiolaria, which form siliceous oozes on the sea floor. As these organisms sink, portions of their shells dissolve near the bottom and reprecipitate in void spaces between shell fragments, resulting in a hard bedded rock.
How are non silicate minerals formed?
Some carbonate rocks, such as calcite and dolomite, are formed via evaporation and precipitation. However, most carbonate-rich rocks, such as limestone, are created by the lithification of fossilized marine organisms.
What is silicon mineral?
Named from the Latin word meaning “flint,” silicon is a shiny, blue-gray metallic substance. It looks like a metal, but its other characteristics are more non-metallic than metallic. It is the second-most common element in the Earth's crust, mostly in the form of silica (SiO2). Pure silicon is never found in nature.
Where does silicon come from?
Silicon makes up 27.7% of the Earth's crust by mass and is the second most abundant element (oxygen is the first). It does not occur uncombined in nature but occurs chiefly as the oxide (silica) and as silicates. The oxide includes sand, quartz, rock crystal, amethyst, agate, flint and opal.
Why are most minerals silicates?
The silicate minerals are the most important mineral class because they are by far the most abundant rock-forming minerals. This group is based on the silica (SiO4) tetrahedron structure, in which a silicon atom is covalently bonded to 4 oxygen atoms at the corners of a triangular pyramid shape.
What are silicates made?
Silicates are salts containing anions of silicon (Si) and oxygen. There are many types of silicates, because the silicon-to-oxygen ratio can vary widely. In all silicates, however, silicon atoms are found at the centres of tetrahedrons with oxygen atoms at the corners.
How are minerals formed?
Minerals can form on the surface through evaporation of solutions containing dissolved minerals. Minerals can form beneath the surface when dissolved elements and compounds leave a hot water solution or when materials melted in magma/ lava then cools & hardens.
What is the difference between a silicate mineral and a non silicate mineral?
Still, two major types of minerals exist: silicates and non-silicates. Silicates are those minerals that have silicon as a component, while non-silicates do not have silicon. As silicates form more than 90% of the earth's crust, we'll start with them.
What is the composition of silicates?
The silicates make up about 95 percent of Earth’s crust and upper mantle, occurring as the major constituents of most igneous rocks.
How many silicate minerals are there?
Of the approximately 600 known silicate minerals, only a few dozen—a group that includes the feldspars, amphiboles, pyroxenes, micas, olivines, feldspathoids, and zeolites —are significant in rock formation. Read More on This Topic. mineral: Silicates. The silicates, owing to their abundance on Earth, constitute the most important mineral class.
What is the basic structural unit of silicate minerals?
The basic structural unit of all silicate minerals is the silicon tetrahedron in which one silicon atom is surrounded by and bonded to (i.e., coordinated with) four oxygen atoms, each at the corner of a regular tetrahedron. These SiO 4 tetrahedral units can share oxygen atoms and be linked in a variety of ways, which results in different structures.
What are nesosilicates made of?
For example, nesosilicates are minerals whose structure are made up of independent silicate tetrahedrons. Sorosilicates are silicate minerals consisting of double tetrahedral groups in which one oxygen atom is shared by two tetrahedrons. Cyclosilicates, in contrast, are arranged in rings made up of three, four, or six tetrahedral units.
What are the most important minerals in igneous rocks?
Most important are beryllium, zirconium, and lithium ,…. …igneous rocks are composed of silicate minerals (meaning that the basic building blocks for the magmas that formed them are made of silicon [Si] and oxygen [O]), but minor occurrences of carbonate-rich igneous rocks are found as well.
What are the void spaces in silicate?
Silicate minerals can be thought of as three-dimensional arrays of oxygen atoms that contain void spaces (i.e., crystallographic sites ) where various cations can enter. Besides the tetrahedral (4-fold coordination) sites, 6-fold, 8-fold, and 12-fold sites are common.
What percentage of the Earth's crust is made up of silicates?
The silicates make up about 95 percent of Earth’s crust and upper mantle, occurring as the major constituents of most igneous rocks and in appreciable quantities in sedimentary and metamorphic varieties as well. They also are important constituents of lunar samples, meteorites, and most asteroids.
What is the structure of silicate?
General structure. A silicate mineral is generally an ionic compound whose anions consist predominantly of silicon and oxygen atoms. In most minerals in the Earth's crust, each silicon atom is the center of an ideal tetrahedron, whose corners are four oxygen atoms covalently bound to it. Two adjacent tetrahedra may share a vertex, ...
How many groups are there in silicate?
In mineralogy, silicate minerals are classified into seven major groups according to the structure of their silicate anion:
How many tetrahedra are in a ring silicate?
Cyclosilicates (from Greek κύκλος kuklos, circle), or ring silicates, have three or more tetrahedra linked in a ring. The general formula is (Si x O 3x) 2x−, where one or more silicon atoms can be replaced by other 4-coordinated atom (s). The silicon:oxygen ratio is 1:3.
What is the most important mineral in the Earth's crust?
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust. In mineralogy, silica (silicon dioxide) SiO 2 is usually considered a silicate mineral. Silica is found in nature as the mineral quartz, and its polymorphs .
What is the biogenic form of silica?
Diatomaceous earth, a biogenic form of silica as viewed under a microscope. The imaged region measures approximately 1.13 by 0.69 mm. Living organisms also contribute to this geologic cycle. For example, a type of plankton known as diatoms construct their exoskeletons ("frustules") from silica extracted from seawater.
Where is the Lunar Ferroan anorthosite found?
Lunar ferroan anorthosite ( plagioclase feldspar) collected by Apollo 16 astronauts from the Lunar Highlands near Descartes Crater. Tectosilicates, or "framework silicates," have a three-dimensional framework of silicate tetrahedra with SiO 2 in a 1:2 ratio. This group comprises nearly 75% of the crust of the Earth.
Where is silica found?
Silica is found in nature as the mineral quartz, and its polymorphs . On Earth, a wide variety of silicate minerals occur in an even wider range of combinations as a result of the processes that have been forming and re-working the crust for billions of years. These processes include partial melting, crystallization, fractionation, metamorphism, ...
Why are silicate minerals so abundant?
The variety and abundance of the silicate minerals is a result of the nature of the silicon atom, and even more specifically, the versatility and stability of silicon when it bonds with oxygen.
How many silicate minerals are there?
Despite the fact that there are many hundreds of silicate minerals, only about 25 are truly common. Therefore, by understanding how these silica tetrahedra form minerals, you will be able to name and identify 95% of the rocks you encounter on Earth's surface.
How do silicates form chains?
The result of this is that the silica tetrahedra can polymerize, or form chain-like compounds, by sharing an oxygen atom with a neighboring silica tetrahedron. The silicates are, in fact, subdivided based on the shape and bonding pattern of these polymers, because the shape influences the external crystal form, the hardness and cleavage of the mineral, the melting temperature, and the resistance to weathering. These different atomic structures produce recognizable and consistent physical properties, so it is useful to understand the structures at an atomic level in order to identify and classify the silicate minerals. Identifying minerals in a rock may seem like an arcane exercise, but it is only by identifying minerals that we begin to understand the history of a given rock.
What is the weathering of silicate minerals on Earth?
The weathering of silicate minerals on the surface of Earth produces the soils in which we grow our foods and the sand on our beaches. The properties of the minerals that are important to us are based on the versatility of the silicate anion in combination with other elements.
Why did Berzelius classify minerals?
Early mineralogists grouped minerals according to physical properties, which spread the silicates across many groups because they have very different properties. By the early 1800s, however, Berzelius had begun classifying minerals based on their chemical composition rather than on their physical properties, defining groups such as the oxides and sulfides – and, of course, the silicates. At the time, Berzelius was able to determine the absolute proportions of elements within a mineral, but he could not see the internal arrangement of the atoms of those elements in their crystalline structure.
What is the term for breakage in crystal structure of certain minerals along planes where atomic bonds are weakest?
Terms you should know. cleava ge : breakage in crystal structure of certain minerals along planes where atomic bonds are weakest. crust : the outermost layer of Earth; the surface layer of a planet. tetrahedron : a figure with four triangular planes; a triangular pyramid. Bookmark.
What is the most common mineral in the Earth's crust?
Plagioclase feldspar is the single most common mineral in Earth's crust, making up an estimated 39% of both continental and oceanic crust. Quartz only makes up an estimated 12% of the entire crust, but it is by far the most common mineral we see on the surface because of its resistance to weathering.
What is the simplest silicate structure?
The simplest silicate structure, that of the mineral olivine, is composed of isolated tetrahedra bonded to iron and/or magnesium ions. In olivine, the –4 charge of each silica tetrahedron is balanced by two divalent (i.e., +2) iron or magnesium cations.
What are the different types of silicates?
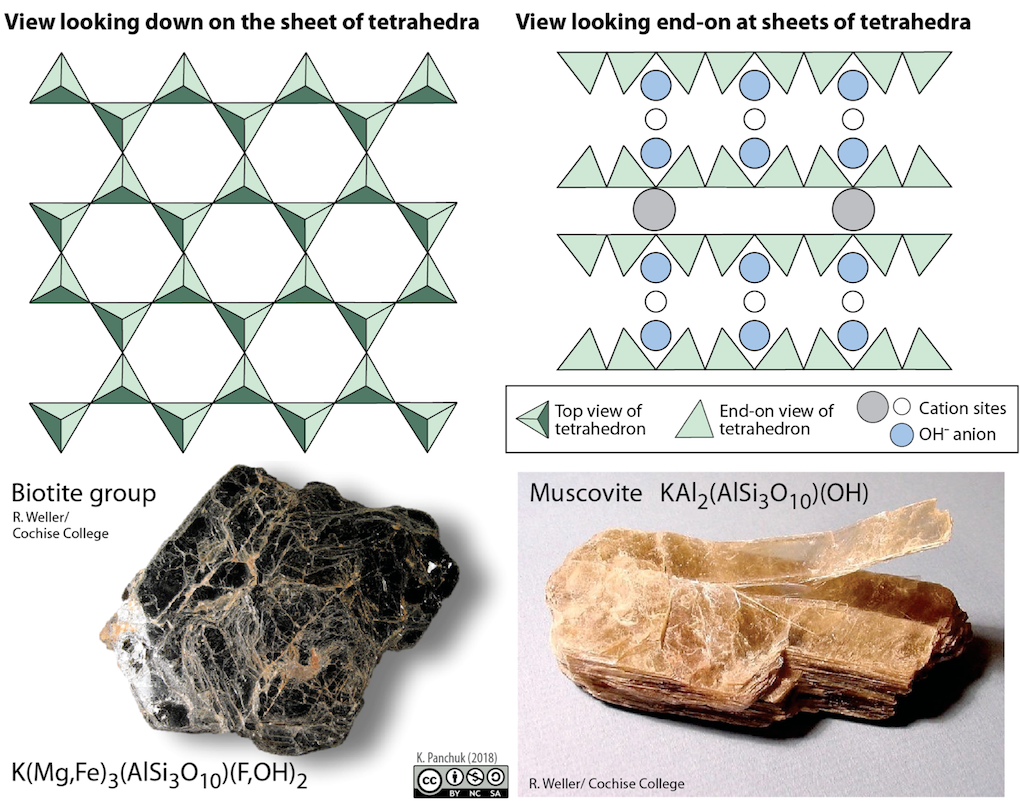
Apart from muscovite, biotite, and chlorite, there are many other sheet silicates (or phyllosilicates ), which usually exist as clay-sized fragments (i.e., less than 0.004 mm). These include the clay minerals kaolinite, illite, and smectite, and although they are difficult to study because of their very small size, they are extremely important components of rocks and especially of soils.
What is the charge of a silica tetrahedron?
Since the silicon ion has a charge of +4 and each of the four oxygen ions has a charge of –2, the silica tetrahedron has a net charge of –4. In silicate minerals, these tetrahedra are arranged and linked together in a variety of ways, from single units to complex frameworks (Figure 2.9). The simplest silicate structure, that of the mineral olivine, ...
How many oxygen ions are in a silica tetrahedron?
Figure 2.13 A single silica tetrahedron (left) with four oxygen ions per silicon ion (SiO4). Part of a single chain of tetrahedra (right), where the oxygen atoms at the adjoining corners are shared between two tetrahedra (arrows). For a very long chain the resulting ratio of silicon to oxygen is 1 to 3 (SiO3).
What are the minerals that make up the Earth's crust?
These include minerals such as quartz, feldspar, mica, amphibole, pyroxene, olivine, and a great variety of clay minerals. The building block of all of these minerals is the silica tetrahedron, a combination of four oxygen atoms and one silicon atom.
What are the ions in magnesium and iron?
As already noted, the +2 ions of iron and magnesium are similar in size (although not quite the same). This allows them to substitute for each other in some silicate minerals. In fact, the common ions in silicate minerals have a wide range of sizes, as shown in Figure 2.11. All of the ions shown are cations, except for oxygen. Note that iron can exist as both a +2 ion (if it loses two electrons during ionization) or a +3 ion (if it loses three). Fe 2+ is known as ferrous iron. Fe 3+ is known as ferric iron. Ionic radii are critical to the composition of silicate minerals, so we’ll be referring to this diagram again.
What are some examples of cyclic silicate minerals?
Examples of cyclic or ring silicate minerals are wollastonite Ca3(Si3O9) and benitoite BaTi (Si3O9).
What is the name of the type of silicate that is formed by joining two chains?
Double chain silicates are also formed, by joining two chains. These type of silicates are called amphiboles.
What is the name of the silicate atom that is neutral?
It is represented as (SiO2)n. When all the four corners oxygen atom of tetrahedra are shared, a three-dimensional network of silica is formed. These type of silicates are neutral.
What is the formula for silicate?
Silicate is an anion consist of silicon and oxygen. Its general formula is (SiO.4-x)n. Silicate mineral is composed of silicate groups. Silica sand or quartz sand is silica ore. These are rock-forming minerals. It consists of SiO4-4 tetrahedra. In silicates only Si-O bonds are present. The hybridization of silicon in silicate is sp3. The Si is present at the centre of the tetrahedra with oxygen occupying the four corners of the tetrahedra.
What is orthosilicate mineral?
Orthosilicates Mineral. It is represented by SiO4-4. These contain discrete SiO4-4 tetrahedra. The corner in Orthosilicates is not shared. Examples of orthosilicate mineral are willemite (Zn SiO4) and olivine (MgSiO4). These type of silicates are formed by metals. Therefore, also known as metal silicates.
What is silicate used for?
It is used in making abrasive material. These materials are used for grinding, polishing, and cleaning. Silicates are used in making prism, eyeglasses, and cuvette. It is used in making different types of laboratory apparatus.
What is the name of the silicate that is joined by oxygen?
In this type of silicate two tetrahedra units are joined together by sharing one oxygen. These are also called island silicates.
What are the most common minerals found in igneous rocks?
Most igneous rocks are dominated by silicate minerals1. However, of the roughly 22,000 minerals known to science, only a very few crystallize from magmas. These are conveniently subdivided into a mafic group, a term that designates those that contain magnesium, ferrous, and possibly ferric iron, and which are all dark-colored minerals (olivine, orthopyroxene, clinopyroxene, hornblende, biotite, garnet, and spinel), and a felsic group, from feldspar and silica, which are all light-colored minerals [quartz, alkali feldspar, plagioclase feldspar and feldspathoid minerals (nepheline, kalsilite, leucite, sodalite), muscovite, and melilite].
What minerals are hydrophobic?
Many hydrophobic silicate minerals such as talc, chlorite, kaolinite and serpentinite activate other minerals by forming slime coatings. It is also known that the serpentine slimes, containing chrysotile and lizardite, form slime coatings on unoxidized pentlandite and depress pentlandite flotation. The formation of these slime coatings is directly related to the magnitude and sign of surface charge of the slimes and sulfide particles.17 Chrysotile was observed to depress nickel sulfide more than lizardite. Addition of small amount of chrysotile to the flotation pulp (0.05 g l − 1) drastically decreases pentlandite flotation recovery, from 90% to 5% ( Figure 13 ).
Is asbestos a mineral?
Asbestos are fibrous silicate minerals (i.e., serpentine and amphibole) that are present in a number of widely used industrial materials (e.g., chrysotile, crocidolite, and amosite) with tensile strength and heat resistance. Occurring secondary to the inhalation of asbestos fibers, asbestosis is a form of diffuse interstitial pulmonary fibrosis that is considered separately from other asbestos-related diseases, such as benign pleural effusion and plaques, malignant mesothelioma, and bronchogenic carcinoma. Histopathologically, asbestos bodies (in the form of a single asbestos fiber surrounded by a segmented protein-iron coat) are readily identifiable in intraalveolar macrophages.
Is a silicate a floatable mineral?
Insoluble oxide and silicate minerals are floatable with both anionic and cationic collectors. The mineral–collector interaction and flotation determined by electrical properties of the mineral surface, electrical charge of the collector, molecular weight of the collector, solubility of the minerals, and stability of metal–collector salts. Depending on the properties, the collector may adsorb either by electrostatic interaction with the surface (physical adsorption) or by specific chemical interaction with surface species (chemical adsorption).
What are Silicate Minerals?
There are thousands of different minerals variations on Earth. Minerals are naturally occurring, inorganic (not made of living things), solid, have a defined chemical composition (unique combination of atoms), and a crystalline structure.
Structure of Silicate Minerals
Silica tetrahedron {eq} (SiO_ {4})^ {4-} {/eq} is the basic building block of all silicate minerals.
Types of Silicates
The basic silicate chemical formula is {eq} (SiO_ {4})^ {4-} {/eq}. Silica minerals are characterized according to how the silica tetrahedral molecules bond to each other and how they bond to other elements.

Overview
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust.
In mineralogy, silica (silicon dioxide) SiO2 is usually considered a silicate mineral. Silica is found in nature as the mineral quartz, and its polymorphs.
General structure
A silicate mineral is generally an ionic compound whose anions consist predominantly of silicon and oxygen atoms.
In most minerals in the Earth's crust, each silicon atom is the center of an ideal silicon–oxygen tetrahedron. Two adjacent tetrahedra may share a vertex, meaning that the oxygen atom is a bridge connecting the two silicon atoms. An unpaired vertex represents an ionized oxygen atom…
Nesosilicates or orthosilicates
Nesosilicates (from Greek νῆσος nēsos 'island'), or orthosilicates, have the orthosilicate ion, which constitute isolated (insular) [SiO4] tetrahedra that are connected only by interstitial cations. The Nickel–Strunz classification is 09.A –examples include:
• Phenakite group
Sorosilicates
Sorosilicates (from Greek σωρός sōros 'heap, mound') have isolated pyrosilicate anions Si 2O 7, consisting of double tetrahedra with a shared oxygen vertex—a silicon:oxygen ratio of 2:7. The Nickel–Strunz classification is 09.B. Examples include:
• Hemimorphite (calamine) – Zn4(Si2O7)(OH)2·H2O
Inosilicates
Inosilicates (from Greek ἴς is [genitive: ἰνός inos] 'fibre'), or chain silicates, have interlocking chains of silicate tetrahedra with either SiO3, 1:3 ratio, for single chains or Si4O11, 4:11 ratio, for double chains. The Nickel–Strunz classification is 09.D – examples include:
• Pyroxene group
• Pyroxenoid group
Phyllosilicates
Phyllosilicates (from Greek φύλλον phýllon 'leaf'), or sheet silicates, form parallel sheets of silicate tetrahedra with Si2O5 or a 2:5 ratio. The Nickel–Strunz classification is 09.E. All phyllosilicate minerals are hydrated, with either water or hydroxyl groups attached.
Examples include:
Tectosilicates
Tectosilicates, or "framework silicates," have a three-dimensional framework of silicate tetrahedra with SiO2 in a 1:2 ratio. This group comprises nearly 75% of the crust of the Earth. Tectosilicates, with the exception of the quartz group, are aluminosilicates. The Nickel–Strunz classifications are 09.F and 09.G, 04.DA (Quartz/ silica family). Examples include:
See also
• Classification of non-silicate minerals – List of IMA recognized minerals and groupings
• Classification of silicate minerals – List of IMA recognized minerals and groupings
• Silicate mineral paint