See more
What is a monatomic hydrogen?
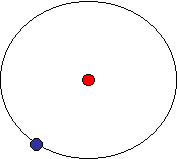
(Image not to scale) A hydrogen atom is an atom of the chemical element hydrogen.
How long does hydrogen have a half life?
Heavier isotopes of hydrogen are only created artificially in particle accelerators and have half-lives on the order of 10 −22 seconds. They are unbound resonances located beyond the neutron drip line; this results in prompt emission of a neutron .
What is the name of the atom that gains a second electron?
If instead a hydrogen atom gains a second electron, it becomes an anion. The hydrogen anion is written as "H – " and called hydride .
What happens when a neutral hydrogen atom loses its electron?
If a neutral hydrogen atom loses its electron, it becomes a cation. The resulting ion, which consists solely of a proton for the usual isotope, is written as "H + " and sometimes called hydron. Free protons are common in the interstellar medium, and solar wind. In the context of aqueous solutions of classical Brønsted–Lowry acids, such as hydrochloric acid, it is actually hydronium, H 3 O +, that is meant. Instead of a literal ionized single hydrogen atom being formed, the acid transfers the hydrogen to H 2 O, forming H 3 O + .
How many neutrons does deuterium have?
Deuterium contains one neutron and one proton in its nucleus. Deuterium is stable and makes up 0.0156% of naturally occurring hydrogen and is used in industrial processes like nuclear reactors and Nuclear Magnetic Resonance .
What did Ernest Rutherford discover about the structure of the atom?
Experiments by Ernest Rutherford in 1909 showed the structure of the atom to be a dense, positive nucleus with a tenuous negative charge cloud around it. This immediately raised questions about how such a system could be stable. Classical electromagnetism had shown that any accelerating charge radiates energy, as shown by the Larmor formula. If the electron is assumed to orbit in a perfect circle and radiates energy continuously, the electron would rapidly spiral into the nucleus with a fall time of:
When was the hydrogen atom solved?
In 1979 the (non-relativistic) hydrogen atom was solved for the first time within Feynman's path integral formulation of quantum mechanics by Duru and Kleinert. This work greatly extended the range of applicability of Feynman's method.

Overview
Isotopes
The most abundant isotope, hydrogen-1, protium, or light hydrogen, contains no neutrons and is simply a proton and an electron. Protium is stable and makes up 99.985% of naturally occurring hydrogen atoms.
Deuterium contains one neutron and one proton in its nucleus. Deuterium is stable and makes up 0.0156% of naturally occurring hydrogen and is used in industrial processes like nuclear reactors
Hydrogen ion
Lone neutral hydrogen atoms are rare under normal conditions. However, neutral hydrogen is common when it is covalently bound to another atom, and hydrogen atoms can also exist in cationic and anionic forms.
If a neutral hydrogen atom loses its electron, it becomes a cation. The resulting ion, which consists solely of a proton for the usual isotope, is written as "H " and sometimes called hydron. …
Theoretical analysis
The hydrogen atom has special significance in quantum mechanics and quantum field theory as a simple two-body problem physical system which has yielded many simple analytical solutions in closed-form.
Experiments by Ernest Rutherford in 1909 showed the structure of the atom to be a dense, positive nucleus with a tenuous negative charge cloud around it. This …
Alternatives to the Schrödinger theory
In the language of Heisenberg's matrix mechanics, the hydrogen atom was first solved by Wolfgang Pauli using a rotational symmetry in four dimensions [O(4)-symmetry] generated by the angular momentum and the Laplace–Runge–Lenz vector. By extending the symmetry group O(4) to the dynamical group O(4,2), the entire spectrum and all transitions were embedded in a single irreducible group representation.
See also
• Antihydrogen
• Atomic orbital
• Balmer series
• Helium atom
• Lithium atom
Books
• Griffiths, David J. (1995). Introduction to Quantum Mechanics. Prentice Hall. ISBN 0-13-111892-7. Section 4.2 deals with the hydrogen atom specifically, but all of Chapter 4 is relevant.
• Kleinert, H. (2009). Path Integrals in Quantum Mechanics, Statistics, Polymer Physics, and Financial Markets, 4th edition, Worldscibooks.com, World Scientific, Singapore (also available online physik.fu-berlin.de)