How do otic quinolones kill bacteria?
Otic quinolones kill bacteriaby preventing DNAsynthesisand replicationin bacterial cells. Otic quinolones work by inhibiting gyrase, an enzymethat is essentialfor bacterial DNA synthesis. Gyrase is a bacterial enzyme that breaks DNA strands, removes knots, repairs and recombines the broken strands to make perfect copies of the bacterial DNA.
Why are quinolones used to treat Mycobacteria?
This drug is a good choice because mycobacteria live inside host cells and are thus hard to reach with many antibiotics. Quinolones and fluoroquinolones inhibit DNA replication by targeting the bacterial enzymes DNA gyrase, aka topoisomerase II, and topoisomerase IV.
How do quinolones kill anaerobes?
6. Conclusions Quinolones are a class of synthetic bactericidal antibiotics with broad-spectrum activity, which can inhibit both Gram-negative and Gram-positive bacteria, including anaerobes. They exert their activity by binding to the bacterial topoisomerase type II enzymes, interfering with the DNA synthesis pathway.
What is the difference between bacteria and quinolones?
Bacterial cells are prokaryotic; primitive cells that differ significantly from humans’ eukaryotic cells. Quinolones exert their bacteriocidal effect by interfering with a bacterium’s ability to make DNA (replication). Article Summary: Antibiotics are chemotherapeutic agents used to inhibit or kill bacteria.

What do quinolones do to stop infection?
Quinolones act by converting their targets, gyrase and topoisomerase IV, into toxic enzymes that fragment the bacterial chromosome.
What is the mechanism of action of quinolones?
As mentioned above, quinolone drugs are active against type II topoisomerases and act by blocking DNA replication and inhibiting synthesis and cell division (Vila, 2005).
How do fluoroquinolones act to destroy bacteria?
Fluoroquinolones act by inhibiting two enzymes involved in bacterial DNA synthesis, both of which are DNA topoisomerases that human cells lack and that are essential for bacterial DNA replication, thereby enabling these agents to be both specific and bactericidal.
What do quinolone antibiotics do?
Quinolone antibiotics are known to work against different bacteria by stopping their ability to grow and infect the cells in the body. This occurs by working against two different enzymes found in bacteria. The enzymes are topoisomerase IV and DNA gyrase. Both interfere with the synthesis of DNA replication.
What is the spectrum of antibacterial action of quinolones?
Quinolones are broad-spectrum antibiotics that are active against both Gram-positive and Gram-negative bacteria, including mycobacteria, and anaerobes.
How do quinolones inhibit gyrase?
Quinolones dually target DNA gyrase and topoisomerase IV binding to specific domains and conformations so as to block DNA strand passage catalysis and stabilize DNA–enzyme complexes that block the DNA replication apparatus and generate double breaks in DNA that underlie their bactericidal activity.
Why are fluoroquinolones so effective in treating bacteria?
These drugs work by targeting 2 bacterial enzymes responsible for notching, coiling, and sealing DNA during replication: DNA gyrase and topoisomerase IV. Because current fluoroquinolones bind to 2 separate enzymes, it's harder for bacteria to mutate and to evade the actions of these drugs.
How do quinolones inhibit DNA synthesis?
The quinolone class of antibiotics inhibits the DNA synthesis of bacteria by disrupting the bacterial topoisomerase type II; inhibiting the catalytic activity of DNA gyrase and topoisomerase IV. These two enzymes are critical bacterial enzymes that regulate the chromosomal supercoiling required for DNA synthesis.
What enzymes do fluoroquinolones inhibit?
The fluoroquinolones interact with 2 bacterial targets, the related enzymes DNA gyrase and topoisomerase IV, both of which are involved in DNA replication. Quinolones form complexes of these enzymes with DNA, complexes that block movement of the DNA-replication fork and thereby inhibit DNA replication.
What class of antibiotic is quinolone?
Quinolones and fluoroquinolones are considered broad-spectrum antibiotics. This means that they are effective against a wide range of bacteria.
Is quinolones bacteriostatic or bactericidal?
Quinolones are chemotherapeutic bactericidal drugs. They interfere with DNA replication by preventing bacterial DNA from unwinding and duplicating.
Are quinolones bactericidal?
Quinolones are a class of antibiotics structurally related to nalidixic acid. They exhibit bactericidal activity primarily by inhibiting bacterial DNA gyrase.
What are the 5 mechanisms of action of antibiotics?
Antimicrobial agents can be divided into groups based on the mechanism of antimicrobial activity. The main groups are: agents that inhibit cell wall synthesis, depolarize the cell membrane, inhibit protein synthesis, inhibit nuclei acid synthesis, and inhibit metabolic pathways in bacteria.
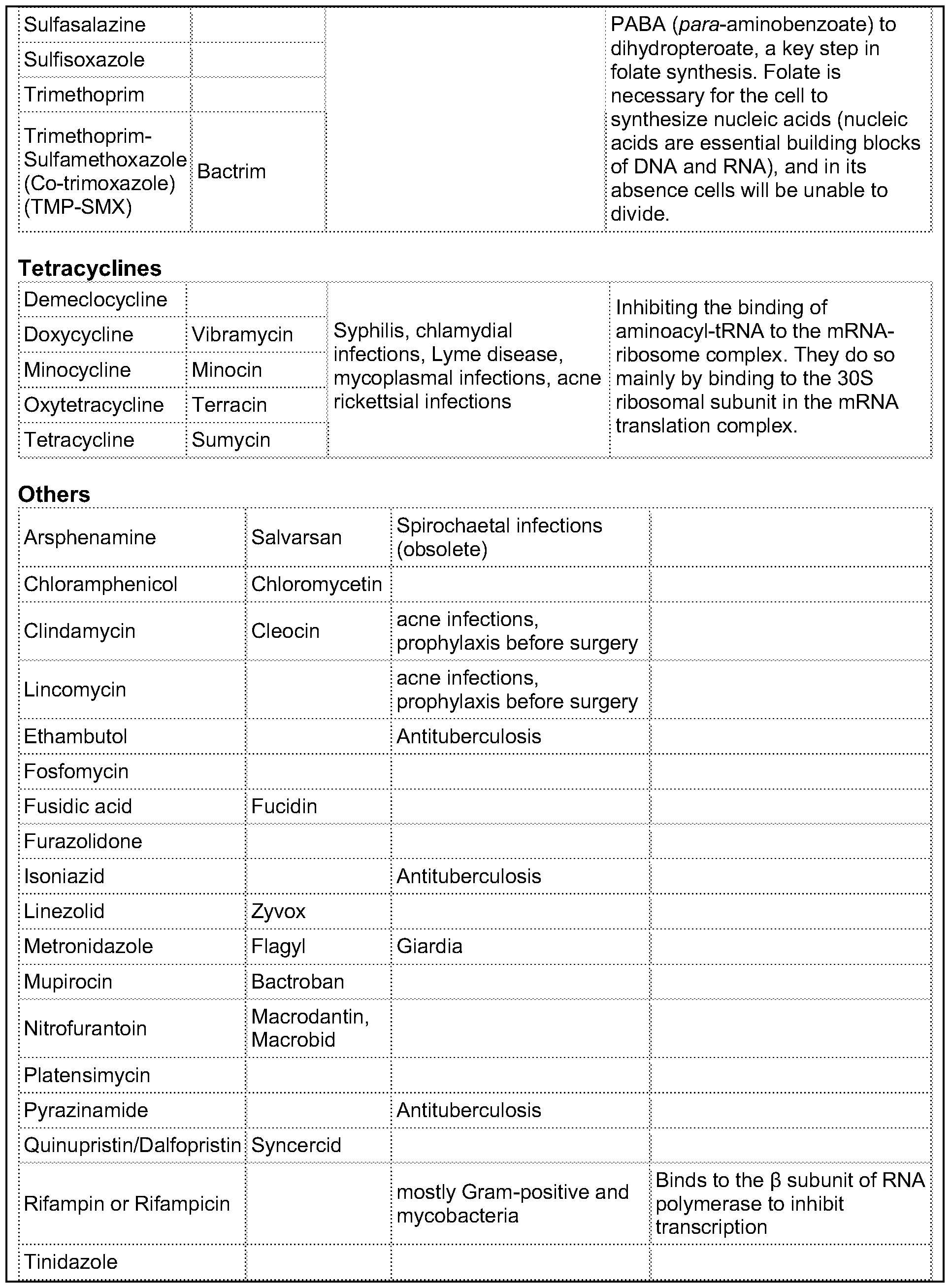
What is the mechanism of action of sulfonamide?
Mechanism of action As a sulfonamide antibiotic, sulfanilamide functions by competitively inhibiting (that is, by acting as a substrate analogue) enzymatic reactions involving para-aminobenzoic acid (PABA). Specifically, it competitively inhibits the enzyme dihydropteroate synthase.
What is the definition of quinolones?
Quinolones are a class of broad-spectrum antibiotics used in the management and treatment of many different bacterial infections.
What is a common side effect of quinolones?
Quinolones have few adverse effects, most notably nausea, headache, dizziness, and confusion. Less common but more serious adverse events include prolongation of the corrected QT interval, phototoxicity, liver enzyme abnormalities, arthropathy, and cartilage and tendon abnormalities.
What is a quinolone antibiotic?
The quinolones are a family of antibiotics containing a bicyclic core structure related to the compound 4-quinolone (Fig. 1).1Since their discovery in the early 1960s, they have gained increasing importance as key therapies to treat both community-acquired and severe hospital-acquired infections.2The first quinolone antibiotic is generally considered to be nalidixic acid, which was reported in 1962 as part of a series of 1-alkyl-1,8-naphthyridines prepared at the Sterling-Winthrop Research Institute.3However, a 2015 perspective that examined the origins of quinolone antibiotics in greater detail points out that the author of the 1962 publication (George Lesher) described the isolation of-chloro-1-ethyl-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid in the late 1950s as a by-product of chloroquine synthesis, with modest antibacterial activity leading to further work on analogues, including nalidixic acid.1Around the same time, Imperial Chemical Industries (ICI) published patent applications with antibacterial quinolones, including a 6-fluoroquinolone.1Nalidixic acid is a narrow-spectrum agent against enteric bacteria used for treating uncomplicated urinary tract infections (UTIs).4During the 1970s–1980s, the coverage of the quinolone class was expanded significantly by the breakthrough development of fluoroquinolones, which show a much broader spectrum of activity and improved pharmacokinetics compared to the first-generation quinolone.5Those fluoroquinolones, such as ciprofloxacin and ofloxacin, are active against both Gram-negative and Gram-positive pathogens; importantly, they are also active against the causative agent of tuberculosis, Mycobacterium tuberculosis. Quinolones have been favoured as antibiotics for more than five decades because of their high potency, broad spectrum of activity, favorable bioavailability, convenient formulations, and high serum concentrations, as well as a comparatively low incidence of side effects.6Quinolones are widely prescribed for several different types of human infections,7with side effects including gastrointestinal reactions, CNS reactions, genotoxicity, phototoxicity, and some minor adverse effects.
What is the review of quinolones?
This review discusses the current knowledge of the development process of quinolones on how structural modifications in the evolving generations have mediated improvements in terms of potency, pharmacokinetics, and toxicity. It also summarizes the relevant knowledge of mode of actions and resistance. Lastly, the review examines future strategies to improve the activity of this class and overcome the resistance.
How do antibiotics inhibit DNA synthesis?
The quinolone class of antibiotics inhibits the DNA synthesis of bacteria by disrupting the bacterial topoisomerase type II; inhibiting the catalytic activity of DNA gyrase and topoisomerase IV.8These two enzymes are critical bacterial enzymes that regulate the chromosomal supercoiling required for DNA synthesis.9Over time, quinolone resistance has become a serious problem among many emerging resistant pathogens.10The mutations generated by the bacteria against quinolones are generally located on the target enzyme binding sites in DNA gyrase and topoisomerase IV.11In addition, resistance to this class of antibiotics can be obtained by acquisition of a resistant plasmid from other sources in the environment through horizontal transfer, leading to the rapid spread of resistance.12
What is the second generation of quinolones?
The first second-generation quinolone, flumequine, exemplified the discovery that a key modification, adding a fluorine (F) atom at the R6position, could significantly improve the spectrum of activity.18This change dramatically increased the quinolone activity, since almost all quinolone antibiotics have been designated as fluoroquinolones, with the exception of the most recent compounds from the fourth generation. Other fluoroquinolones from the second generation include enoxacin, norfloxacin, and ciprofloxacin, which were able to inhibit all Gram-negative organisms, including Pseudomonasspecies.19In addition to the fluoro substituent, these drugs were further modified by addition of a piperazine ring to the R7position and addition of a cyclopropyl group to the R1position. The R7piperazine ring improved the Gram-negative potency,20while the cyclopropyl group was found to improve the overall activity of the compounds.21This combination made ciprofloxacin the most active compound among the early compounds of the second generation and made it the first choice used against Pseudomonas aeruginosatoday.22Subsequent development of the second generation produced analogues with activity against some Gram-positive bacteria, including Staphylococcus aureusbut not Streptococcus pneumoniae, and some atypical organisms (Mycoplasma pneumoniaeand Chlamydia pneumoniae).23The presence of an alkylated piperazine group at the R7position, as in ofloxacin, marked the first modifications that help inhibit Gram-positive organisms.24The addition of an –OCH3substituent to the R8position of the latter group also helped to improve Gram-positive activity.25Of all compounds in this latter group (2b), ofloxacin is considered as the most powerful as it combines all the new substituents and it is now still being used for clinical treatment. Ofloxacin is a chiral molecule and its l-isomer is the only active compound, which is known as levofloxacin. It was proposed to have 4-fold higher activity compared with ofloxacin and is also more active than ciprofloxacin in treating some strains.26,27
Is quinolone a Gram negative?
The first-generation quinolone activity was limited to only Gram-negative organisms, excluding Pseudomonasspecies.16Shortly after the clinical introduction of nalidixic acid, it was found to cause rapid resistance development in a number of organisms, reducing its effectiveness17and leading to investigations to discover analogues with improved properties.
What is quinolone antibiotic?
Quinolones are a class of broad-spectrum antibiotics used in the management and treatment of many different bacterial infections. This activity reviews the indications, contraindications, mechanism of action, adverse events, and other critical elements of quinolone therapy in the clinical setting pertinent for members of the healthcare team in managing the care of patients with bacterial infections treated with quinolones and its potential sequelae.
What is quinolone used for?
Quinolones are a class of broad-spectrum antibiotics used in the management and treatment of many different bacterial infections. This activity reviews the indications, contraindications, mechanism of action, adverse events, and other critical elements of quinolone therapy in the clinical setting pe …
What are quinolones used for?
Type of Infections Quinolones are Used Against. Clinical applications of quinolones include the treatment of common urinary tract infections, and certain gastrointestinal infections, gynecological infections / STDs, skin infections, and upper and lower respiratory infections (such as sinusitis, pneumonia, bronchitis).
Why is it so difficult for bacteria to multiply?
Since a copy of DNA must be made each time a cell divides, interfering with replication makes it difficult for bacteria to multiply. How DNA is packaged is very different in bacteria as opposed to eukaryotes. Bacteria supercoil DNA using DNA gyrase, whereas eukaryotes coil DNA around histone proteins.
What is the purpose of antibiotics?
Article Summary: Antibiotics are chemotherapeutic agents used to inhibit or kill bacteria. But how do quinolones destroy these microbes without hurting our cells? MOA of Quinolone Antibiotics.
Can quinolone cause nausea?
Adverse Effects of Quinolones. Quinolones typically have few side effects, most commonly including nausea, headache, dizziness, and confusion. Rare but serious adverse events have been reported, such as prolongation of the corrected QT interval, phototoxicity, liver enzyme abnormalities, arthropathy (joint problems), ...
What is the main target of quinolones?
The major target of quinolones and fluoroquinolones, especially in Gram-negative bacteria, is the enzyme DNA gyrase, which is also known as topoisomerase II. This enzyme normally relieves torsional stress during DNA replication. What does that mean? Well, as the replication fork moves along the bacterial chromosome, the strand of DNA in front of it becomes supercoiled, or excessively twisted. DNA gyrase binds to the DNA, cuts one of the strands, and allows it to untwist a bit before resealing the strand. But when quinolones or fluoroquinolones are present, DNA gyrase is inhibited and cannot reseal the DNA strands. This causes the bacterial chromosome to break into small fragments, and this extensive DNA damage kills the bacterium.
What enzyme kills bacteria?
This causes the bacterial chromosome to break into small fragments, and this extensive DNA damage kills the bacterium. In Gram-positive bacteria, the major target of quinolones and fluoroquinolones is a related enzyme called topoisomerase IV.
What enzymes inhibit DNA replication?
Quinolones and fluoroquinolones inhibit DNA replication by targeting the bacterial enzymes DNA gyrase, aka topoisomerase II, and topoisomerase IV. DNA gyrase untwists the DNA during replication to relieve torsional stress, and topoisomerase IV cuts the daughter chromosomes apart after replication. When these important enzymes are inhibited by antibiotics, DNA breakage occurs and the bacteria die because of the extensive DNA damage. The quinolone nalidixic acid is often used to treat bladder infections, and the fluoroquinolone ciprofloxacin is used to treat many infections, including anthrax.
What is the best rifamycin for mycobacterial infections?
The best-known and most effective member of the rifamycin family is rifampin , which is also known as rifampicin. A major use of rifampin is in the treatment of mycobacterial diseases, such as tuberculosis and leprosy. Since mycobacteria are obligate intracellular bacteria, they live within host cells, where they're protected against many antibiotics that can't get inside. Rifamycins can penetrate well into cells and tissues, so they're a good first choice for mycobacterial infections. However, as with any antibiotic, there are bacteria that are resistant to the rifamycins. The most common way for bacteria to become resistant to rifamycins is to acquire mutations that alter the structure of the RNA polymerase in such a way that rifamycins can't bind to it as well.
What are the two primary inhibitors of DNA and RNA synthesis?
The two primary inhibitors of DNA and RNA synthesis are rifamycins and quinolones. Discover how the antibodies known as rifamycins, quinolones, and fluoroquinolones kill bacteria in this lesson. Updated: 09/20/2021
Why are rifamycins effective?
Rifamycins are broad-spectrum antibiotics, meaning they're effective against many types of bacteria, including Gram-negative, Gram-positive, and obligate intracellular bacteria. There are two main reasons for this. First, the rifamycin molecule can penetrate well into cells and tissues. This means that, unlike some antibiotics that can't cross certain types of bacterial cell walls, the rifamycins can almost always get in and gain access to their target enzyme. And second, the bacterial RNA polymerase is well-conserved even among very different bacteria. This means that the enzyme's structure is similar enough that the rifamycins can bind well to their target in diverse types of bacteria.
Why does topoisomerase IV die?
When topoisomerase IV is inhibited by these antibiotics, the same result occurs: the bacterium dies because of DNA breakage. All this talk about bacterial death lets us know that quinolones and fluoroquinolones are bactericidal antibiotics.
How do otic quinolones work?
Otic quinolones are broad spectrum antibiotic medications that are used to treat bacterial infections in the external and middle ear. Otic quinolones kill bacteria by preventing DNA synthesis and replication in bacterial cells. Otic quinolones work by inhibiting gyrase, an enzyme that is essential for bacterial DNA synthesis.
How are otic quinolones used?
Otic quinolones are solutions or suspensions that are topically administered into the ear canal. Otic quinolones are used to treat or prevent infections in the following conditions or treatment procedures:
How does quinolone kill bacteria?
The ability of quinolone antibiotics to kill bacteria is a function of the stable interaction complex formed between drug-bound topoisomerase enzyme and cleaved DNA 4. Mechanistically, based on studies employing DNA cleavage mutants of gyrase 23 and topoIV 24 that do not prevent quinolone binding, as well as studies that have shown that strand breakage can occur in the presence of quinolones 25, it is accepted that DNA strand breakage occurs after the drug has bound the enzyme. Therefore, the net effect of quinolone treatment is to generate double-stranded DNA breaks that are trapped by covalently (yet reversibly) linked topoisomerases whose functions are compromised 26 – 28. As a result of quinolone-topoisomerase-DNA complex formation, DNA replication machinery becomes arrested at blocked replication forks, leading to inhibition of DNA synthesis, which immediately leads to bacteriostasis and eventually cell death 4 ( Figure 1a ). It should be noted, however, that these effects on DNA replication can be correlated with bacteriostatic concentrations of quinolones, and are regarded as being reversible 4, 29. Nonetheless, considering that gyrase has been found to be distributed approximately every 100 kilobases along the chromosome 30, poisoning of topoisomerases by quinolone antibiotics and the resulting formation of stable complexes with DNA have substantial, negative consequences for the cell in terms of its ability to deal with drug-induced DNA damage 31.
What is the role of quinolone antibiotics in DNA supercoiling?
a) Quinolone antibiotics interfere with changes in DNA supercoiling by binding to topoisomerase II or IV. This leads to the formation of double-stranded DNA breaks and cell death in either a protein synthesis dependent or protein synthesis independent fashion.
What is the role of quinolones in chromosomes?
The quinolone class of antimicrobials interferes with the maintenance of chromosomal topology by targeting DNA gyrase (topoisomerase II) and topoisomerase IV (topoIV), trapping these enzymes at the DNA cleavage stage and preventing strand rejoining 4, 19, 20 ( Figure 1a ). Despite the general functional similarities between topoIV and gyrase, the susceptibility of these targets to quinolone antibiotics varies across bacterial species 20 (Table 1). For example, several studies have shown that topoIV is the primary target of quinolones in Gram-positive bacteria (e.g., Streptococcus pneumoniae 21 ), whereas gyrase is the primary target and topoIV the secondary target of these drugs in Gram-negative bacteria (e.g., E. coli 13 and Neisseria gonorrhoea 22 ).
How does DNA break in quinolones affect DNA stress?
The introduction of double-stranded DNA breaks following topoisomerase inhibition by quinolones induces the DNA stress response (SOS response), in which RecA is activated by DNA damage and promotes auto-cleavage of the LexA repressor protein , inducing expression of SOS-response genes including DNA repair enzymes 32. Notably, several studies have shown that preventing induction of the SOS response serves to enhance killing by quinolone antibiotics (except in the case of the first generation quinolone, nalidixic acid) 8, 33. Preventing induction of the SOS response has also been shown to reduce the formation of drug-resistant mutants by blocking the induction of error-prone DNA polymerases 34, homologous recombination 20, and horizontal transfer of drug-resistance elements 35, 36.
What is the process of bacterial cell death?
Antibiotic-mediated cell death, however, is a complex process that begins with the physical interaction between a drug molecule and its bacterial-specific target, and involves alterations to the affected bacterium at the biochemical, molecular and ultrastructural levels.
When were quinolones first used?
Quinolones are derivatives of nalidixic acid, which was discovered as a byproduct of chloroquine (quinine) synthesis and introduced in the 1960s to treat urinary tract infections 16. Nalidixic acid and other first generation quinolones (i.e., oxolinic acid) are rarely used today owing to their toxicity 17.
When was penicillin first discovered?
Since the discovery of penicillin was reported in 1929 2, other, more effective antimicrobials have been discovered and developed by elucidation of drug-target interactions, and by drug molecule modification. These efforts have significantly enhanced our clinical armamentarium.
