
To calculate an electron configuration, divide the periodic table into sections to represent the atomic orbitals, the regions where electrons are contained. Groups one and two are the s-block, three through 12 represent the d-block, 13 to 18 are the p-block and the two rows at the bottom are the f-block.
Full Answer
How do you find the electronic structure of an atom?
The electronic structure of an atom can be predicted from its atomic number. For example, the atomic number of sodium is 11. Sodium atoms have 11 protons and so 11 electrons: This electronic structure can be written as 2,8,1 (each comma, or dot, separates one shell from the next). This electronic structure can also be shown as a diagram.
What is an electronic structure?
An electronic structure. is the way in which electrons. are arranged in an atom. Electrons in shells Electrons in atoms occupy energy levels, also called electron shells, outside the nucleus .
What are the methods of electronic structure calculations?
Electronic structure calculations aim to numerically solve the Schrodinger equation for a system with only the basic description provided. Some popular electronic structure methods include DFT[8–10], Hartree–Fock (HF) method [11,12], Quantum Monte Carlo [13–15], coupled cluster [16,17], multireference configuration interaction, etc.
How are elements arranged in the modern periodic table?
In the modern periodic table, elements are in order of atomic number in periods and groups. Electronic structures model how electrons are arranged in atoms. An electronic structure is the way in which electrons are arranged in an atom. Electrons in atoms occupy energy levels, also called electron shells, outside the nucleus.
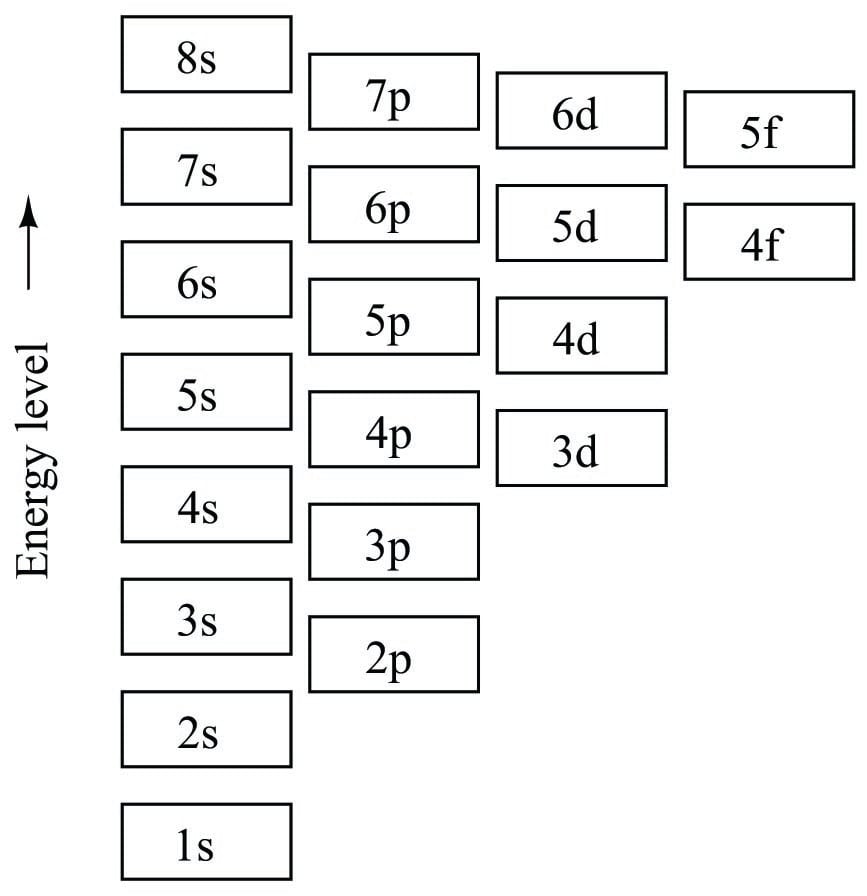
How is electronic structure calculated?
Electronic structure is obtained by solving quantum mechanical equations for the aforementioned clamped-nuclei problem. Electronic structure problems arise from the Born–Oppenheimer approximation.
What do you mean by electronic structure?
electronic configuration, also called electronic structure or electron configuration, the arrangement of electrons in orbitals around an atomic nucleus.
What is an electronic structure in number form?
An electronic structure is the way in which electrons are arranged in an atom....Electronic structure and the periodic table.Electronic structure featureLink to the periodic tableNumber or numbers of circlesPeriod numberNumber of electrons in outermost shellGroup numberTotal number of electronsAtomic number
Why is electronic structure important?
A short and simplified answer: The electronic configuration of an atom determines the chemical reactions the atom can participate in, and determines the kinds of molecules that atoms can combine into to form more complicated substances.
What is the electronic structure of water?
The molecule H2O has ten electrons associated, eight from the oxygen atom and one each from the two hydrogen atoms. Its electronic structure has been proposed as 1sO2.00 2sO1.82 2pxO1.50 2pzO1.12 2pyO2.00 1sH10.78 1sH20.78 [71], with the inner 1sO pair of electrons unhybridized.
How do you draw electronic structures GCSE?
2:426:24GCSE Chemistry - Electron Arrangement #8 - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd to do that we would just do two comma eight comma eight to show there's two electrons in theMoreAnd to do that we would just do two comma eight comma eight to show there's two electrons in the first shell eight in the second. And eight in the third.
How do you draw the electronic structure of an ion?
0:4111:05Electron Configuration of Ions - Mg2+, P3-, Fe2+, Fe3+ - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo first it's 1s2. And then we'll move on to 2s. So this is going to be 2s2. And then after 2s it'sMoreSo first it's 1s2. And then we'll move on to 2s. So this is going to be 2s2. And then after 2s it's 2p then 3s p can hold up to 6. And then 3s2.
What is the 2 8 8 electron rule?
Transition metals are able to put more than eight electrons in the shell that is one in from the outermost shell. Think about argon (Ar). It has 18 electrons set up in a 2-8-8 order. Scandium (Sc) is only 3 spots away with 21 electrons, but it has a configuration of 2-8-9-2.
What are the electronic structures of atoms?
Electrons in an atom are grouped around the nucleus into shells. Shell (electron): A grouping of electrons in an atom according to energy. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons.
Who discovered the electronic structure?
Joseph John “J. J.” ThomsonIn 1897 Thomson discovered the electron and then went on to propose a model for the structure of the atom. His work also led to the invention of the mass spectrograph. The British physicist Joseph John (J. J.)
What is the electronic structure of carbon?
[He] 2s2 2p2Carbon / Electron configuration
What is electronic structure of lithium?
1s²2s¹Lithium / Electron configuration
How does Mk level change with electronegativity?
The Mk level correlates with the electronegativity of M, as shown in Fig. 6-5A. For nontransition metals denoted by open circles , Mk levels change linearly with the electronegativity. For transition metals denoted by solid circles, they are located well above a dotted line of nontransition metals. This indicates that the existence of 3 d electrons affects the energy of 4 s electrons. It is known that the energy eigenvalue obtained by the DV-Xα calculation represents the electronegativity [14], although the interaction between neighboring atoms in a cluster necessitates some modification of this idea for a free atom. In Fig. 6-5A, a line of slope of –1 is drawn as a reference, since the M-s level (eV) may be expressed using the electronegativity Φ (eV) as, M -s level=−Φ+c, where c is an arbitrary constant. This line is located between the transition metals and nontransition metals.
What is the relationship between Mk and atomic radius?
Also, the Mk level is associated with the atomic radius of M as shown in Fig. 6-5B. The Mk level increases with increasing atomic radius of elements, although there are some differences in the correlation between transition metals and nontransition metals. Thus, the s -orbital energy level, Mk, is obtained for the nontransition metal, Al, that is different from the d -orbital energy level, Md, for the transition metals such as Ni, Fe, and Ti. However, both the Mk and the Md parameters correlate with the electronegativity and the atomic radius of elements. These parameters reflect the nature of chemical bond between atoms in the alloys. The mechanical properties of Al alloys are predictable using the Mk parameter as explained later.
Where are the 4s electrons written?
Notice in what follows that all the 3-level orbitals are written together - with the 4s electrons written at the end of the electronic structure.
Which electron governs the properties of the elements?
The outer electron governs their properties and that electron is in the same sort of orbital in both of the elements. That wouldn't be true if the outer electron in potassium was 3d1.
How many D electrons are there?
Remember that there are five d orbitals, and that the electrons will inhabit them singly as far as possible. Up to 5 electrons will occupy orbitals on their own. After that they will have to pair up.
How many electrons are in the outer level of helium?
The number of outer electrons is the same as the group number. (The noble gases are a bit of a problem here, because they are normally called group 0 rather then group 8. Helium has 2 outer electrons; the rest have 8.) All elements in group 3, for example, have 3 electrons in their outer level.
Why are the 2p and 3p electrons lumped together?
The logic is that the 3p electrons will be involved in bonding because they are on the outside of the atom, whereas the 2p electrons are buried deep in the atom and aren't really of any interest.
What is the short cut for a p electron?
Shortcut 1: All the various p electrons can be lumped together. For example, fluorine could be written as 1s22s22p5, and neon as 1s22s22p6.
What is the structure of chlorine?
On this basis the structure of chlorine would be written [Ne]3s23px23py23pz1.
What is electronic potential?
The electronic potential is a function of nuclear coordinates. In Molecular Dynamics, the nuclei move along this energy surface obeying Newton’s Laws of Motion.
What is computational chemistry?
Computational Chemistryis a branch of chemistry that uses computer science to assist in solving chemical problems. Incorporates the results of theoretical chemistry into efficient computer programs. Application to single molecule, groups of molecules, liquids or solids. Calculates the structure and properties of interest. Computational Chemistry Methods range from
What is the PES of a molecule?
The potential energy surface (PES) is multi-dimensional (3N 6 for non-linear molecule and 3N 5 for linear molecule) The PES contains multiple minima and maxima. Geometry optimization search aims to find the global minimum of the potential surface. Transition state or saddle point search aims to find the maximum of this potential surface, usually along the reaction coordinate of interest.
How to write a wavefunction?
1Wavefunction is written as a single determinant = det(˚
How is energy minimization done?
Energy minimization is done when using either molecular mechanics or quantum mechanics methods, and it must precede any computational analyses in which these methods are applied. For example, geometry optimization can be used to.
How many categories of theoretical chemistry are there?
Theoretical Chemistry can be broadly divided into two main categories
Which theory is based on the idea that nuclei are heavier than electrons?
Ab Initio Theory . Born-Oppenheimer Approximation: Nuclei are heavier than electrons and can be considered stationary with respect to electrons. Also know as "clamped nuclei" approximations and leads to idea of potential surface Slater Determinants: Expand the many electron wave function in terms of Slater determinants.
