Follow these steps to find the molarity of an unknown solution with the titration method:
- Prepare the concentrations – Put the analyte in an Erlenmeyer flask and the titrant in a burette.
- Mix the concentrations – Add the titrant to the analyte until the endpoint is reached. You can find this moment by observing the color change. Use the acid-base indicator for this purpose. ...
- Calculate the molarity – Use the titration formula. ...
How do you calculate the average molarity during titration?
How do you calculate molar concentration from titration? Divide the number of moles of analyte present by the original volume of the analyte . For example, if the original volume of the analyte was 500 mL, divide by 1000 mL per L to obtain 0.5 L. Divide 0.01 moles of analyte by 0.5 L to obtain 0.02 moles per liter.
How to calculate molarity in resulting solution?
To calculate molarity, divide the number of moles of solute by the volume of the solution in liters. If you don't know the number of moles of solute but you know the mass, start by finding the molar mass of the solute, which is equal to all of the molar masses of each element in the solution added together.
How do I find the molarity after mixing?
Calculate the final molarity of the mixed solution using the equation molarity = moles ÷ liter. For the example, the final molarity is 0.0135 moles ÷ 0.170 liters = 0.079 M.
How do you calculate normality from molarity?
Steps
- Gather information about the equivalent weight of the reacting substance. Consult chemical reference books to find out the valence and the molecular weight of the substance.
- Figure the equivalent weight of the substance. The equivalent weight of the substance is equal to the molecular weight divided by the valence.
- Calculate normality. ...
- Try an example. ...

How do you calculate after dilution?
2:526:13Dilution Problems - Chemistry Tutorial - YouTubeYouTubeStart of suggested clipEnd of suggested clipWe can plug these values into our equation in order to solve for the volume of our first solution.MoreWe can plug these values into our equation in order to solve for the volume of our first solution. We need to divide each side by 2.5 molar this allows units of molar to cancel out and we're left with
How do you calculate molarity when water is added?
0:362:48What is the molarity of water? - YouTubeYouTubeStart of suggested clipEnd of suggested clip1 000 grams divided by the molar mass of water. You can calculate the molar mass by adding two h'sMore1 000 grams divided by the molar mass of water. You can calculate the molar mass by adding two h's to an o you're using the masses from the periodic.
How do you calculate the molarity of a solution dissolved in water?
The key to calculating molarity is to remember the units of molarity (M): moles per liter....To calculate molarity:Find the number of moles of solute dissolved in solution,Find the volume of solution in liters, and.Divide moles solute by liters solution.
How do you calculate the molarity of a liquid solution?
The most common way to express solution concentration is molarity (M), which is defined as the amount of solute in moles divided by the volume of solution in liters: M = moles of solute/liters of solution.
What is the molarity of 1 Litre of h2o?
Thus, the molarity of pure water is 55.56 moles per litre.
How do you calculate molality of water?
With the help of the density of pure water, the number of moles of the water can be calculated. The density of pure water at room temperature i.e., 25∘C is 0.9970749 g/mol, therefore the mass will be 0.9970749 kg, and the molecular mass is 18.0148 g/mol. The molality is 55.510 m.
What is the molarity of 1l of water?
55.55 MFrom this, the mass of the water is 1000 g in the volume of 1 Liter of solution. So the Molarity of pure water is 55.55 M.
Why is the molarity of water 55?
1 Answer. Show activity on this post. We know that the mass of 1000 mL of water is 1000 g, and the molecular weight of water is around 18 gmol−1, therefore the calculation gives us an answer of 55.5 mol.
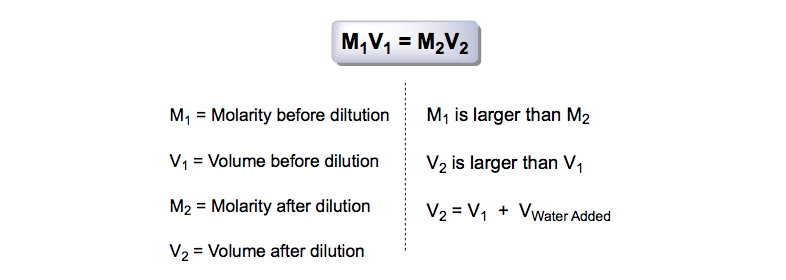
What is the notation for M1V1?
An alternative and commonly-used notation for this equation is M1V1 = M2V2, where M is used in place of C.
What is V1 in chemistry?
V1 is the volume to be removed (i.e., aliquoted) from the concentrated stock solution.
When will relevant comments and/or instructions appear?
Relevant comments and/or instructions will appear here after a calculation is performed.
What is molarity and dilution?
Dilution is a way of decreasing the concentration of a solution. Both molarity and dilution are essential concepts for correctly performing chemical experiments in a laboratory.
How much sodium hydroxide is in a mole?
The “moles” cancel out, and we end up with 20.0 grams of sodium hydroxide.
How many molars does 0.500 L have?
So the equation for molarity is that x divided by 0.500 L equals 1.00 molar, where “x” is the number of moles of sodium hydroxide we want in our solution.
What is the calibration mark for sodium hydroxide?
Once the sodium hydroxide has completely dissolved, we add distilled water until the total volume of the solution reaches the 500. mL calibration mark on the neck of the volumetric flask.
What is a dilution in chemistry?
A dilution is where we start out with a more concentrated stock solution---which is a solution with a high molarity ---and we try to create a solution with a lower molarity.
What is in one glass of salt?
In one glass, we put a small amount of sodium chloride which is table salt.
Why do we use distilled water in aqueous solutions?
When we make an aqueous solution, we want to use distilled water because distilled water is free from any impurities that might affect the making of the solution.
How to find molarity of a solution?
Find the molarity by calculating the number of moles of the solute dissolved in liters of a solution.
What is molarity in math?
He holds bachelor's degrees in both physics and mathematics. Molarity is a unit of concentration, measuring the number of moles of a solute per liter of solution. The strategy for solving molarity problems is fairly simple. This outlines a straightforward method to calculate the molarity of a solution.
What is molarity?
Molarity expresses the concentration of a solution. It is defined as the number of moles of a substance or solute, dissolved per liter of solution (not per liter of solvent!).
What is the molarity of a solution?
molarity = concentration / molar mass. The concentration denotes the mass concentration of the solution, expressed in units of density (usually g/l or g/ml). Molar mass is the mass of 1 mole of the solute. It is expressed in grams per mole. It is a constant property of each substance - for example, the molar mass of water is approximately equal ...
How to find molarity of a molecule?
Convert the expressions above to obtain a molarity formula. As mass / volume = molarity * molar mass, then mass / (volume * molar mass) = molarity .
How to find the concentration of an unknown solution?
Titration is a technique with which you can find the concentration of an unknown solution, based on its chemical reaction with a solution with a known concentration. This process is based on adding the titrant (with a known concentration & volume) to a known quantity of the unknown solution (the analyte) till the reaction is complete. You can then determine the concentration of the analyte by measuring the volume of titrant used.
What is the mole of a substance?
According to the newest conventions (effective as of the 20 th May 2019), the mole definition is that a mole is the amount of a chemical substance that contains exactly 6.02214076*10²³ particles, such as atoms, molecules, ions etc. That number is known as Avogadro's constant. It's symbol is NA or L.
How many grams is carbon 12?
It follows that the molar mass of carbon-12 is exactly 12 grams per mole, M (¹²C) = 12 g/mol. The word "substance" in the definition should specify (be replaced with the name of) the substance concerned in a particular application, e.g., the amount of chloride (HCl) or the amount of carbon dioxide (CO₂). It is crucial to always give a precise specification of the entity involved (as noted in the second part of the mole definition). This should be done by providing the empirical chemical formula of the compound involved.
How much is the molar mass of hydrochloric acid?
Find the molar mass of your substance. For the hydrochloric acid, it is equal to 36.46 g/mol.
How to identify a dilution solution?
You can identify a dilution solution by the amount of solute in the total volume, expressed as a proportion. For example, a chemical may be prepared in a 1:10 dilution of alcohol, indicating that a 10 mL bottle contains one milliliter of chemical and nine milliliters of alcohol. You can calculate the necessary volume of each component to prepare a dilution solution.
How many milliliters of alcohol are in a 10 ml bottle?
For example, a chemical may be prepared in a 1:10 dilution of alcohol, indicating that a 10 mL bottle contains one milliliter of chemical and nine milliliters of alcohol. You can calculate the necessary volume of each component to prepare a dilution solution.
How to convert a dilution factor to a fraction?
Convert the dilution factor to a fraction with the first number as the numerator and the second number as the denominator. For example, a 1:20 dilution converts to a 1/20 dilution factor.
Who is Regina Edwards?
Regina Edwards has been a freelance writer since 1990. She has penned video scripts, instructional manuals, white papers and abstracts. She has also ghostwritten diabetes journals. Edwards is a scuba instructor and Usui and Karuna Reiki teacher. She holds a Bachelor of Science from Saint Joseph's University.