How do you calculate the dilution ratio?
Let's discover the maths and science behind calculating the dilution ratio. solvent volume = solute volume * 5. As you probably noticed, there are 5 different variables in these calculations: Solute ratio - which is always equal to 1 in our dilution ratio calculator; Final volume - this is the desired amount of diluted liquid you want to get;
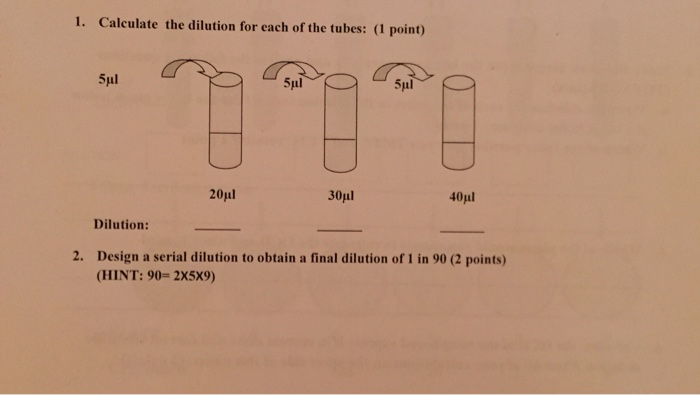
What is the dilution factor for the first tube?
For the first tube, dilution factor = 10 -1 (1 ml added to 9 ml) For the second tube, dilution factor = 10 -1 (1ml added to 9 ml) = total dilution of 10 -1 × 10 -1 = 10 -2
How many tubes does it take to dilute a cell?
As six tubes are used, the final dilution for the bacteria/cells will be 10 -6 (1 in 1,000,000). Serial dilution is performed in a number of experimental sciences like biochemistry, pharmacology, physics, and homeopathy.
What is the procedure for a ten-fold dilution of a sample?
The following is the procedure for a ten-fold dilution of a sample to a dilution factor of 10 -6: The sample/culture is taken in a test tube and six test tubes, each with 9 ml of sterile diluents, which can either be distilled water or 0.9% saline, are taken. A sterile pipette is taken.

What is the formula for calculating dilution?
The formula for calculating a dilution is (C1) (V1) = (C2) (V2) where... C1 is the concentration of the starting solution. V1 is the volume of the starting solution. C2 is the concentration of the final solution.
How do you calculate water dilution?
This process is known as dilution. We can relate the concentrations and volumes before and after a dilution using the following equation: M₁V₁ = M₂V₂ where M₁ and V₁ represent the molarity and volume of the initial concentrated solution and M₂ and V₂ represent the molarity and volume of the final diluted solution.
How do you calculate dilution in microbiology?
Dilution = amount of specimen transferred divided by the [amount of specimen transferred + amount already in tube]. But after the first tube, each tube is a dilution of the previous dilution tube.
How do you solve dilution problems?
3:1621:55Dilution Problems, Chemistry, Molarity & Concentration ... - YouTubeYouTubeStart of suggested clipEnd of suggested clipWe have the new concentration. It's 0.20 we also have the final volume it's 500.. So now let's findMoreWe have the new concentration. It's 0.20 we also have the final volume it's 500.. So now let's find v1. The first thing we need to do is multiply 0.2 by 500. And that's equal to 100.
How do you do a simple dilution?
0:119:16AS Biology - How to calculate serial and simple dilutions - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo each time there is a times 10 dilution factor okay so from 10 to 1 it's gone it's been diluted 10MoreSo each time there is a times 10 dilution factor okay so from 10 to 1 it's gone it's been diluted 10 times and then from 1 to 0.1. Also it's been diluted 10 times so this is a serial dilution.
What is the dilution factor of tube 3?
1 : 100So, tube #3 has 1 / (10 x 10) dilution which is 1 / 100 or 1 : 100. Now, we can take 1 ml from tube #3 and place it into tube #4.
How do you calculate CFU per mL?
To find out the number of CFU/ ml in the original sample, the number of colony forming units on the countable plate is multiplied by 1/FDF. This takes into account all of the dilution of the original sample. ... 200 CFU x 1/1/4000 = 200 CFU x 4000 = 800000 CFU/ml = 8 x 10.CFU/ml in the original sample.
How do you do a 1/10 dilution?
For example, a 1:10 dilution is a mixture of one part of a solution and nine parts fresh solvent. For a 1:100 dilution, one part of the solution is mixed with 99 parts new solvent. Mixing 100 µL of a stock solution with 900 µL of water makes a 1:10 dilution.
What is serial dilution?
A more exact serial dilution definition is that it is a stepwise dilution of a solution, that is repeated a certain number of times and in which the concentration decreases with each step. The dilution factor calculator at each step does not have to be constant, but it is for this calculator.
Why do scientists use serial dilutions?
Serial dilutions are a common practice in the natural sciences. Due to the period decrease in concentration, this method is very useful when performing many types of experiments, from chemistry to biology to medicine.
How many units of volume are in a 1:3 dilution?
If you have a 1:3 dilution, i.e. a 1:3 dilution ratio, this means that you add 1 unit volume of solute (e.g., concentrate) to 3 unit volumes of the solvent (e.g., water), which will give a total of 4 units of volume.
How to mix cleaner concentrate?
Calculate the concentrate volume (solute volume). solute volume = final volume / (solvent ratio + solute ratio)
