
How to Draw a Lewis Dot Structure Step 1. Determine the total number of valence electrons to be depicted in the Lewis diagram. Example: CO 2 Total = 16 Step 2. Place least electronegative element in center and draw single bonds from the central atom to other atoms.
How do you write a triple bond in Lewis structure?
A triple bond forms when three electron pairs are shared by a pair of atoms, as in carbon monoxide (CO) and the cyanide ion (CN – ): For very simple molecules and molecular ions, we can write the Lewis structures by merely pairing up the unpaired electrons on the constituent atoms.
Why is a covalent bond represented with a dash instead of dots?
It is common to represent the covalent bond with a dash connecting the two elemental symbols, instead of with two dots: Because two atoms are sharing one pair of electrons, this covalent bond is called a single bond. As another example, consider fluorine.
What is the Lewis dot diagram of N in NH3?
The N atom has the following Lewis electron dot diagram: It has three unpaired electrons, each of which can make a covalent bond by sharing electrons with an H atom. The electron dot diagram of NH3 is as follows:
Why is hydrogen a covalent bond and not a molecular bond?
Because each H atom has a filled valence shell, this bond is stable, and we have made a diatomic hydrogen molecule. It is common to represent the covalent bond with a dash connecting the two elemental symbols, instead of with two dots:
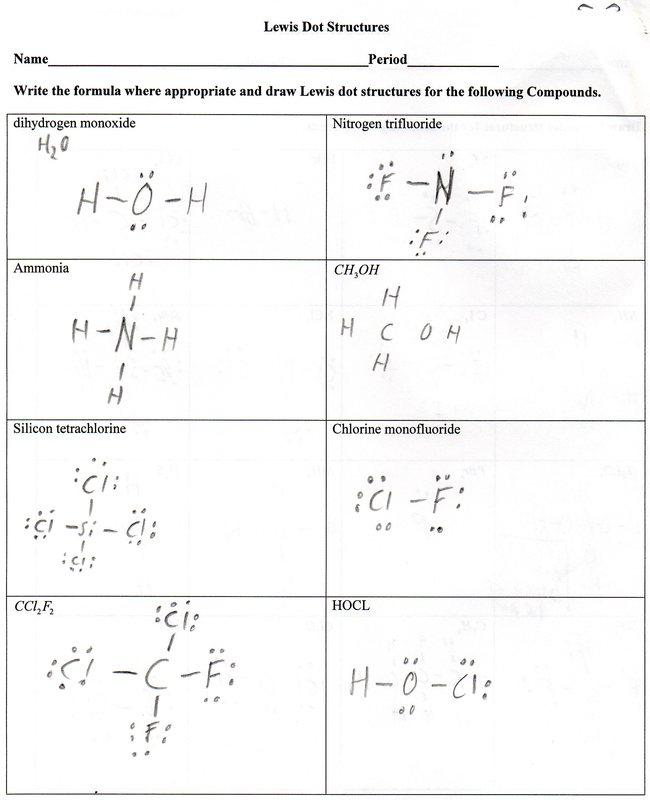
How do you draw a covalent bond step by step?
Step 1: Add up the total valence electrons. Step 2: Determine the central atom. Step 3: Place non-central atoms around the central atom, and connect using lines to represent the bonds. Step 4: Distribute remaining electrons around the non-central atoms for a full valence shell.
What is the first step to drawing a covalent Lewis dot structure?
Step 1: Determine the total number of valence electrons. Step 2: Write the skeleton structure of the molecule. Step 3: Use two valence electrons to form each bond in the skeleton structure. Step 4: Try to satisfy the octets of the atoms by distributing the remaining valence electrons as nonbonding electrons.
How do you draw Lewis structures step by step?
3:277:26Lewis Diagrams Made Easy: How to Draw Lewis Dot Structures - YouTubeYouTubeStart of suggested clipEnd of suggested clipFirst count all of the valence electrons in the molecule. For water each hydrogen has one electronMoreFirst count all of the valence electrons in the molecule. For water each hydrogen has one electron and we multiply that by two because there are two hydrogens. In the molecule.
How do you draw a Lewis dot structure?
Determine which atom will be the central atom of the Lewis Dot Structure. The central atom is the least most electronegative atom in the compound. Remember the trend for electronegativity on the periodic table. Once determined, draw that element by atomic symbol in the center and draw single bonds to the other atoms.
How many unpaired electrons does NH3 have?
The N atom has the following Lewis electron dot diagram: It has three unpaired electrons, each of which can make a covalent bond by sharing electrons with an H atom. The electron dot diagram of NH3 is as follows:
How many electrons does fluorine have?
As another example, consider fluorine. F atoms have seven electrons in their valence shell: These two atoms can do the same thing that the H atoms did; they share their unpaired electrons to make a covalent bond.
Why is the H atom a diatomic molecule?
Because each H atom has a filled valence shell, this bond is stable, and we have made a diatomic hydrogen molecule. It is common to represent the covalent bond with a dash connecting the two elemental symbols, instead of with two dots:
What do you use to show covalent bonding in PCL 3?
Use a Lewis electron dot diagram to show the covalent bonding in PCl 3.
How many atoms can form a covalent bond?
More than two atoms can participate in covalent bonding, although any given covalent bond will be between two atoms only. Consider H and O atoms: The H and O atoms can share an electron to form a covalent bond:
When do atoms share electrons?
As previously mentioned, when a pair of atoms shares one pair of electrons, we call this a single bond. However, a pair of atoms may need to share more than one pair of electrons in order to achieve an octet. A double bond forms when two pairs of electrons are shared between a pair of atoms, as between the carbon and oxygen atoms in CH 2 O (formaldehyde) and between the two carbon atoms in C 2 H 4 (ethylene):
What type of bond forms when three electrons are shared by a pair of atoms?
A triple bond forms when three electron pairs are shared by a pair of atoms, as in carbon monoxide (CO) and the cyanide ion (CN – ):
