
How is free energy related to equilibrium constant?
Free Energy and the Equilibrium Constant. At equilibrium, the forward and reverse reactions proceed at equal rates. The driving force in each direction is equal, because the free energy of the reactants and products under equilibrium conditions is equivalent (ΔG = 0). We also know that, at equilibrium, Q = Keq.
How is free energy determined at equilibrium?
The balance between reactants and products in a reaction will be determined by the free energy difference between the two sides of the reaction. The greater the free energy difference, the more the reaction will favor one side or the other.
How is free energy calculated?
The binding free energy can be calculated using the rate constants kon and koff as ΔG=Gbound-Gunbound=-kTln KeqC0=-kTln C0konkoff, where Keq is the binding equilibrium constant, C0 is the reference concentration of 1 mol/L, k is Boltzmann's constant and T is the temperature in Kelvin.
What is the free energy of equilibrium?
At equilibrium, the instantaneous difference in free energy between reactants and products is zero, which means there's no more driving force for the reaction. And at equilibrium, the reaction quotient Q is equal to the equilibrium constant K. So we can plug in zero for delta G in our equation.
Why is Gibbs free energy 0 at equilibrium?
If the difference in Gibbs energy for the forward reaction is G, so the change in Gibbs energy is -G for the backward reaction. This is why energy from Gibbs is zero at equilibrium.
What is r in G =- Rtlnk?
In general: ΔG = ΔG° + RTlnQ. R = the gas constant = 8.314 J/mol·K. T = temperature in K. Q = reaction quotient.
How do you calculate delta G from equilibrium constant?
1:304:48How to Calculate Keq from Delta G - YouTubeYouTubeStart of suggested clipEnd of suggested clipRight we have ke Q equals V to the power of negative Delta G over RT. That's e to the power ofMoreRight we have ke Q equals V to the power of negative Delta G over RT. That's e to the power of negative. Now the gas constant I'm familiar with this 8.314. And it is in joules per mole Kelvin. So. I
What is free energy of a reaction?
The standard free energy change (∆Gº') of a chemical reaction is the amount of energy released in the conversion of reactants to products under standard conditions.
What is the value of ∆ G when a system is at equilibrium?
ΔG = 0If a system is at equilibrium, ΔG = 0. If the process is spontaneous, ΔG < 0. If the process is not spontaneous as written but is spontaneous in the reverse direction, ΔG > 0.
What is Q in Gibbs free energy?
Using Standard Change in Gibbs Free Energy, ΔG° where R is the ideal gas constant 8.314 J/mol K, Q is the reaction quotient, and T is the temperature in Kelvin.
What is N in Gibbs free energy equation?
The total charge transferred from the reductant to the oxidant is therefore nF, where n is the number of moles of electrons.
What is the free energy change for a reaction at equilibrium?
We also said that at equilibrium, Q, the reaction quotient is equal to the equilibrium constant, K. And we plugged K into the equation and solved for delta-G. Delta-G was equal to zero. So, we know, at equilibrium, the change in free energy is equal to zero.
What happens to Gibbs free energy at equilibrium?
GIbbs free energy is a measure of how much potential a reaction has left to do a net something. So the free energy is zero at equillibrium and no more work can be done.
How does the Gibbs function relate to the equilibrium stage?
The relation between Gibbs energy and equilibrium constant is △Go=−RTlnKeq. The relation between Gibbs energy and change in enthalpy is △G= △H- T△S. Note: The beauty of △G= △H- T△S is the ability to determine the relative importance of the enthalpy and entropy as driving forces behind a reaction.
Is free energy maximum at equilibrium?
A reaction proceeds towards the direction of lesser Gibbs free energy (at constant T (temperature) and P (pressure)). So, we could say that Gibbs free energy at equilibrium is minimum.
Free Energy and Equilibrium - Key takeaways
Gibb's free energy, or just Free energy, is the amount of available energy in a system to do work. The formula for the change in free energy (Δ G) is:
Gibbs Free energy and the equilibrium constant
As a reaction proceeds, the Free energy (G) is going to decrease. This is because the "available energy" of the system is being used up. In experiments, we cannot measure the Free energy directly, but we can only measure the change in Free energy, ΔG. The change in Free energy will continue to decrease until it reaches a minimum, ΔG = 0.
Derivation of relation between Gibbs free energy and equilibrium constant
We can use ΔG° to calculate K eq and vice-versa. Before we get started, we first need to understand the concept of the reaction quotient.
Equation for Gibbs free energy and equilibrium constant
Now that we have our equation, what does it tell us? It shows us that the relationship between free energy and K eq is inversely proportional. This means that if one increases, the other decreases. We can rearrange our equation to solve for K eq:
Thermodynamics
Thermodynamics studies the relationships between heat, work, temperature, and energy. It describes how the energy of a system changes and whether or not that energy can be used for useful work.
GIBBS FREE ENERGY
Gibbs free energy, or free energy, is the maximum amount of energy a substance can contribute to a chemical transformation or reaction.
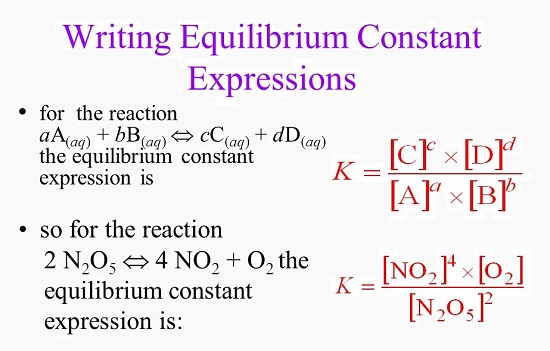
EQUILIBRIUM CONSTANT
The equilibrium constant is the ratio of the concentration of products to the concentration of reactants.
RELATION BETWEEN GIBBS FREE ENERGY AND EQUILIBRIUM CONSTANT
When equilibrium is reached, there is no additional free energy change, i.e., ΔG = 0, and Q is equal to the equilibrium constant (K (eq)). Hence the relationship between K (eq) and ΔG becomes:
SOURCE
Gibbs Free Energy: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_ (Physical_and_Theoretical_Chemistry)/Thermodynamics/Energies_and_Potentials/Free_Energy/Gibbs_ (Free)_Energy. Accessed 28 Feb 2022
Thermodynamics
Gibbs Free Energy
- Gibbs free energy, or free energy, is the maximum amount of energya substance can contribute to a chemical transformation or reaction.
- It is equal to the sum of entropy and the product of temperature in a closed system.
- In simpler words, it is the sum of entropy and enthalpy.
- The change in energy, ΔG, can indicate the direction of chemical reaction under two conditio…
- Gibbs free energy, or free energy, is the maximum amount of energya substance can contribute to a chemical transformation or reaction.
- It is equal to the sum of entropy and the product of temperature in a closed system.
- In simpler words, it is the sum of entropy and enthalpy.
- The change in energy, ΔG, can indicate the direction of chemical reaction under two conditions: constant pressure and constant temperature.
Equilibrium Constant
- The equilibrium constant is the ratio of the concentration of products to the concentration of reactants.
- It is denoted by K(eq).
- The formula of the equilibrium constant is:
Relation Between Gibbs Free Energy and Equilibrium Constant
- When equilibrium is reached, there is no additional free energy change, i.e., ΔG = 0, and Q is equal to the equilibrium constant (K(eq)). Hence the relationship between K(eq) and ΔG becomes: ΔGo = –RT In K(eq) or ΔGo = –2.303 RT log K(eq) 1. Where R is the ideal gas constant (8.314 J/K mol), 2. T is the Kelvin temperature, 3. And K(eq) is the natur...
FAQ’s
- 1. What is the relationship between free energy and equilibrium constant? When equilibrium is reached, there is no additional free energy change, i.e., ΔG = 0, and Q is equal to the equilibrium constant (K(eq)). Hence the relationship between K(eq) and ΔG becomes: ΔGo = –RT In K(eq), orΔGo = –2.303 RT log K(eq) 2. How do you calculate free energy? Calculation of Free energy:Δ…
Source
- Gibbs Free Energy: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Ma…
- Calculations of Free Energy: https://flexbooks.ck12.org/cbook/ck-12-chemistry-flexbook-2.0/section/20.8/primary/lesson/calculations-of-free-energy-and-keq-chem/. Accessed 28 Feb 2022.