
To find oxidation numbers, figure out if the substance in question is elemental or an ion. If it’s elemental, it has an oxidation number of 0. If it’s an ion, its oxidation number is the same as its charge. Be aware that metallic ions that can have more than one charge, like iron, can also have more than one oxidation number!
What are the rules for oxidation numbers?
What are the rules for determining oxidation numbers and examples?
- The oxidation number of a free element is zero.
- The oxidation number of a monatomic ion equals the charge on the ion.
- The sum of oxidation numbers in a neutral compound is zero. ...
- The oxidation number of the Group IA element is +1 and Group IIA element is +2,
How do you assign oxidation numbers?
Oxidation number or oxidation state of an atom or ion in a molecule/ion is assigned by: i) Summing up the constant oxidation state of other atoms/molecules/ions that are bonded to it and. ii) Equating, the total oxidation state of a molecule or ion to the total charge of the molecule or ion.
How to solve oxidation numbers?
half reactions!
- Assign oxidation numbers to determine what is oxidized and what is reduced.
- Write the oxidation and reduction half-reactions.
- Balance each half-reaction: a.Balance elements other than H and O. ...
- Multiply the half-reactions by integers so that the electrons gained and lost are the same.
- Add the half-reactions, subtracting things that appear on both sides.
How to find the oxidation number of an element?
Using Lewis diagrams
- Draw the Lewis diagram for the compound, including all valence electrons.
- Assign the electrons from each bond to the more negative bond partner identified by ionic approximation. Homonuclear bonds should be divided equally.
- The resulting atom charges then represent the oxidation state for each atom.
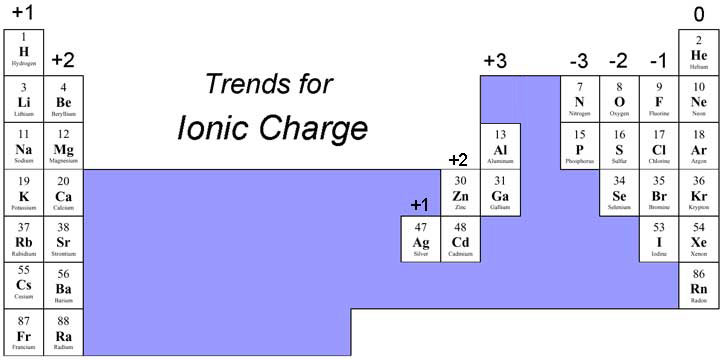
How do you determine the oxidation number?
1:2931:15How To Calculate Oxidation Numbers - Basic Introduction - YouTubeYouTubeStart of suggested clipEnd of suggested clipState of each oxygen atom in this ion. You could write an equation as two oxygen atoms with a totalMoreState of each oxygen atom in this ion. You could write an equation as two oxygen atoms with a total charge of negative two. So individually each oxygen atom has a charge of minus one.
How do you find the oxidation number of an ion?
0:061:34How to Find Oxidation Numbers for an Ion - YouTubeYouTubeStart of suggested clipEnd of suggested clipNumber the chlorine in the center that's normally minus one except when it's bonded to things likeMoreNumber the chlorine in the center that's normally minus one except when it's bonded to things like well chlorine bromine iodine and then oxygen.
What are oxidation numbers in chemistry?
oxidation number, also called oxidation state, the total number of electrons that an atom either gains or loses in order to form a chemical bond with another atom.
What is the oxidation number of O2?
zero oxidationAnd thus, we can say that atoms in their elemental state, even when bonded to an identical atom, are neither reduced nor oxidized and so have a zero oxidation number. So, what is the oxidation number of oxygen in O2? The answer is zero.
What is the oxidation number for h2o?
It is important to note that oxidation number always refers to each individual atom in the compound, not to the total for that element. For example, in H2O, the total positive "charge" for both hydrogen atoms will be +2 (which balances with the -2 from oxygen), but each hydrogen has an oxidation number of +1.
How do you find the oxidation number of molecules and ions?
1:047:00How to Find Oxidation Numbers (Rules and Examples) - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo to solve the equation x minus four equals zero x that equals positive four and that's theMoreSo to solve the equation x minus four equals zero x that equals positive four and that's the oxidation. Number on the carbon. In co2. If we add all these oxidation numbers up we have 2 times -2.
How do you assign oxidation numbers to ions?
0:295:59Assigning Oxidation Numbers - Chemistry Tutorial - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo elements in group 1a. And 2a would have oxidation numbers of plus 1 and plus 2 respectively. AndMoreSo elements in group 1a. And 2a would have oxidation numbers of plus 1 and plus 2 respectively. And aluminum is assigned an oxidation number of plus 3.
What are the rules of oxidation number?
Rules for Assigning Oxidation NumbersThe convention is that the cation is written first in a formula, followed by the anion. ... The oxidation number of a free element is always 0. ... The oxidation number of a monatomic ion equals the charge of the ion. ... The usual oxidation number of hydrogen is +1.More items...•
How can you find the oxidation number of an ion Labster?
1:5416:47Redox Reactions - Discover how batteries work! (Labster Simulation)YouTubeStart of suggested clipEnd of suggested clipThe oxidation number is the charge on an atom if all bonds were fully ionic. In the periodic. TableMoreThe oxidation number is the charge on an atom if all bonds were fully ionic. In the periodic. Table element based on the periodic. Table what is the oxidation number of oxygen in compounds.