How do you determine the stability of chair conformations?
When drawing chair conformations, the type of bond alternates. If carbon one is axial, then carbon two is equatorial. Some chair conformations are more stable than the others and therefore preferred. A key factor for determining stability is to calculate the 1,3-diaxial strain.
Why are some chair conformations preferred over others?
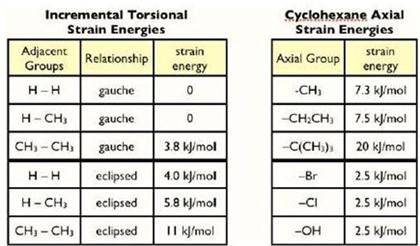
Some chair conformations are more stable than the others and therefore preferred. A key factor for determining stability is to calculate the 1,3-diaxial strain. This is a type of steric interaction that occurs when bulky attachments are located on axial bonds.
Is the chair conformation ring flip hard to draw?
The logic and reasoning behind the chair conformation ring flip will be discussed in my upcoming chair conformations video series. (stay tuned) However, drawing the ring flip doesn't have to be as hard as students think. Yes the flip happens when one molecule changes its conformation to another, but the key to drawing the flip successfully is to…
What is the most stable chair conformation of compound 1?
I am asked to draw the most stable chair conformation of compound 1. After doing so I am a little uncertain which of the conformations is the most stable. The most stable conformation is the one where the most bulky group is positioned equatorial.

Which chair conformation is most stable?
The most stable conformation of cyclohexane is the chair form shown to the right. The C-C-C bonds are very close to 109.5o, so it is almost free of angle strain. It is also a fully staggered conformation and so is free of torsional strain.
How do you know what the most stable conformation is?
To find the most stable conformation, we choose the form with the least number of large axial groups; the least stable will have the most number of axial groups.
Why the chair conformation is relatively stable?
Answer: Chair conformation of cyclohexane is more stable than boat form because in chair conformaion the C-H bonds are equally axial and equatorial, i.e., out of twelve C-H bonds, six are axial and six are equatorial and each carbon has one axial and one equatorial C-H bond.
How do you know if axial or equatorial is more stable?
Study NotesA conformation in which both substituents are equatorial will always be more stable than a conformation with both groups axial.When one substituent is axial and the other is equatorial, the most stable conformation will be the one with the bulkiest substituent in the equatorial position.
What is a stable conformation?
These conformations differ by the relative positions of the two methyl substituents. In the most stable conformation, the two methyl groups lie as far apart from each other as possible with a dihedral angle of 180 degrees. This particular staggered conformation is called anti.
Which conformation is more stable and why?
Staggered conformation of ethane is most stable while eclipsed conformation is least stable because staggered form has the least torsional strain and the eclipsed form has the maximum torsional strain.
Why is half chair conformation least stable?
The half chair, formed by raising the footrest of the chair, has five of the six C atoms in a plane and one C atom out of the plane. Therefore, it has both eclipsing and bond angle strains and hence is the least stable conformation of cyclohexane.
Is chair or boat more stable?
The chair conformation is more stable than the boat conformation. The boat conformation can sometimes be more stable than it is usually, by a slight rotation in the C-C bonds and is called the skew boat conformation. Nevertheless, the chair conformation is the most stable cyclohexane form.
Which of the following confirmation has highest stability?
Correct answer is (b) Chair conformationAll India Exams.KVPY.
Which chair conformation is more stable axial or equatorial?
5:5024:40Chair Conformation and Ring Flips - YouTubeYouTubeStart of suggested clipEnd of suggested clipGroup now let's rank the stability of the four confirmations that we see here. So we're going to sayMoreGroup now let's rank the stability of the four confirmations that we see here. So we're going to say that number one is the most stable number four is the least stable. So which one is going to be the
Which cyclohexane is more stable?
The most stable conformation of cyclohexane is the chair form shown to the right. The C-C-C bonds are very close to 109.5o, so it is almost free of angle strain. It is also a fully staggered conformation and so is free of torsional strain.
How do you determine axial and equatorial positions?
0:295:49Axial vs Equatorial Position (Part I) - YouTubeYouTubeStart of suggested clipEnd of suggested clipPosition either converts to this chair conformation the axial methyl group becomes equatorial methylMorePosition either converts to this chair conformation the axial methyl group becomes equatorial methyl group so the axial becomes equatorial and the equatorial becomes.
Organic Chemistry
In the previous two posts, we have talked about drawing the ring-flip of chair conformations and the A value (1,3-diaxial interactions). And we learned that for a given cyclohexane, the axial conformer is less stable than the corresponding equatorial conformer.
Alkanes and Cycloalkanes
In the previous two posts, we have talked about drawing the ring-flip of chair conformations and the A value (1,3-diaxial interactions). And we learned that for a given cyclohexane, the axial conformer is less stable than the corresponding equatorial conformer.
CORE Concepts
Covered in Other Articles
- Newman Projections
- Fischer Projections
- Naming Cycloalkanes
What Are Chair Conformations?
- Chair conformations are the most stable conformations cyclohexane can form. The basic structure is shown below. Each point represents carbon. To help visualize, it should be noted that carbon 1 is pointing up above the plane and carbon 4 is pointing down below the plane. Every other carbon lies in the same plane. Moreover, the conformation continuously jumps back and f…
Stability and Ring Flips
- Some chair conformations are more stable than the others and therefore preferred. A key factor for determining stability is to calculate the 1,3-diaxial strain. This is a type of steric interactionthat occurs when bulky attachments are located on axial bonds. To calculate the 1,3-diaxial strain, you must add the strain energies of substituents loca...
Further Reading