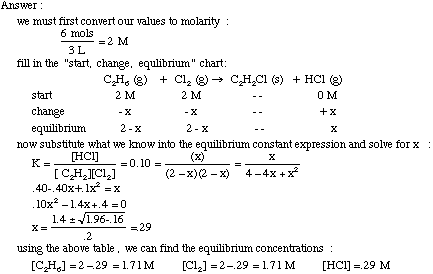
How can You Tell if a Reaction is Reversible?
- A reversible reaction is a simultaneous conversation of reactants into products and products into reactants.
- An example of a reversible reaction is H 2 g + I 2 g ⇌ 2 HI
How do you identify a reversible reaction?
You can look at a reversible reaction as being made up of two separate reactions: The reaction in which the reactants react to make the products is known as the forward reaction. The reaction in which the products react to make the reactants is down as the reverse reaction or the backward reaction.
What is the equilibrium point of a reversible reaction?
Reversible reactions will reach an equilibrium point where the concentrations of the reactants and products will no longer change. A reversible reaction is denoted by a double arrow pointing both directions in a chemical equation. For example, a two reagent, two product equation would be written as A + B ⇆ C + D
How does energy change in a reversible reaction?
Energy changes in reversible reactions. If a reaction is exothermic in one direction, it will be endothermic in the other direction. The same amount of energy is transferred in both the forwards and reverse reaction.
What is a thermic reversible reaction?
A reversible reaction is a reaction where the reactants and products react together to give the reactants back. A and B can react to form C and D or, in the reverse reaction, C and D can react to form A and B. One example of a reversible reaction is the reaction of hydrogen gas and iodine vapor to from hydrogen iodide. Thermic means heat.
How do you tell if a reaction is reversible?
If a reaction can go in both the forward and reverse direction, it's a reversible reaction, which we indicate with two single-headed arrows. But if a reaction can only go in the forward direction, it's an irreversible reaction, which we can indicate in our chemical equation by using a single double-headed arrow.
What makes a reaction reversible?
A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur simultaneously. A and B can react to form C and D or, in the reverse reaction, C and D can react to form A and B.
How do you know if a reaction will go forward or reverse?
Q can be used to determine which direction a reaction will shift to reach equilibrium. If K > Q, a reaction will proceed forward, converting reactants into products. If K < Q, the reaction will proceed in the reverse direction, converting products into reactants. If Q = K then the system is already at equilibrium.
What makes a chemical reaction irreversible?
If a reaction cannot take place in the reverse direction, i.e, the products formed do not react to give back the reactants under the same condition is called an irreversible reaction.
What are 4 examples of reversible reactions?
Examples of Reversible ReactionReaction between hydrogen (H2) and iodine (I2) to produce hydrogen iodide (HI). ... Nitrogen (N2) reacting with hydrogen (H2) to produce ammonia (NH3). ... Sulfur dioxide (SO2) reacts with oxygen (O2) to make sulfur trioxide (SO3)More items...
Which reaction is not reversible?
When one of the products of a reaction is removed from the chemical equilibrium system as soon as it is produced, the reverse reaction cannot establish itself and equilibrium is never reached. Reactions such as these are said to go to completion. These processes are often referred to as non-reversible reactions.
Are all reactions reversible?
In principle, all chemical reactions are reversible reactions . This means that the products can be changed back into the original reactants .
What is the difference between reversible and irreversible reaction?
A reversible reaction proceeds in both directions but an irreversible reaction occurs only in forward direction.
Which statement is always true about a reversible chemical reaction?
This is Expert Verified Answer the amount of reactants and product do not change is always true for a chemical reaction that has reached equilibrium.
What are 5 examples of irreversible change?
Some examples of irreversible changes are burning of paper, Burning of fuels (like Wood, Coal and LPG), Cooking of food, Rusting of iron , Grinding of wheat grains into flour, Baking of chapatti (roti), Growth of a plant, Formation of flower from bud, Falling of leaves from a tree, Ripening of fruits, Ageing of man and ...
Which of the following is a reversible reaction?
Melting, boiling, evaporation, freezing, condensation, and disintegration are examples of reversible transformations. Melting wax, freezing ice, and boiling water that evaporates as steam and condenses back to water are a few examples.
Which of the following is a characteristic feature of reversible reaction?
We can understand from the above explanation that a characteristic feature of a reversible reaction is that it will never precede towards completion. Therefore, the correct answer is option [A] they never proceed to completion.
How can a reversible reaction be irreversible?
So, by removing the product it is possible to make the reversible reaction irreversible as this condition will always shift the equation in the forward direction.
What is the difference between reversible and irreversible reactions?
(2) Reversible processes can take place either in forward direction or in backward direction. (2) Irreversible processes can take place only in one direction.
What is a reversible reaction GCSE?
What is a reversible reaction? A reversible reaction is one that can also happen in reverse! So not only can the reactants react to form the products, but the products can also react to reform the reactants. The double arrow means that the reaction is reversible.
Which of the following is a characteristic feature of reversible reaction?
We can understand from the above explanation that a characteristic feature of a reversible reaction is that it will never precede towards completion. Therefore, the correct answer is option [A] they never proceed to completion.
What is a reversible reaction?
A reversible reaction is a chemical reaction in which the reactants form products, which in turn can react to form the reactants once again.
Which of the following are reversible reactions?
Dissolving a salt
What is the name of the reaction that goes from reactants to products in a reversible reaction?
The forward reaction
What is the name of the reaction that goes from products to reactants in a reversible reaction?
The backward reaction
Which of the following is true about a dynamic equilibrium?
The rate of the forward reaction and the rate of the backward reaction is the same.
Which of the following is true about a dynamic equilibrium?
The concentrations of reactants and products stay the same.
What is the equilibrium constant?
A value expressing the ratio of reactants to products in a dynamic equilibrium.
Why are chemical reactions reversible?
This is because the quantum theory of chemical reactions is too complex to solve theoretically.
What is a reversible reaction?
So a reversible reaction is one that when completed in the reactor (at the specified conditions) will be some mixture of products and reagents. An irreversible one is one that will be virtually 100% when finished.
How many jules does 2H2 release?
Thus 2H2 (g)+O2 (g)⟶2H2O (l) releases 237.13 kilo Jules per mole of thermal energy. The reverse reaction would require this amount of energy to be added to drive the Rxn to completion, and its deltaG would be +237.13 kJ/mol.
How do you reverse a chemical reaction?
Reversible chemical reactions are typically those that involve breaking and forming just a few bonds; these reactions are in a true equilibrium state, and you can make them reverse by perturbing that equilibrium to push the reaction in the other direction. The more comm
What is irreversibility in science?
The concept of irreversibility is really a chemical engineering one . It means that a reaction tends to go so far in one direction that for practical purposes it is one way. For example hydrogen burns with oxygen to make water.
How to get a reaction to go backwards?
You can get the reaction to go backwards by either supplying something (such as copper (II) ions) that is more attractive to carbonate than calcium ions, or by supplying enough acid to convert all the carbonate into carbonic acid.
What does it mean when a reaction goes in one direction?
It means that a reaction tends to go so far in one direction that for practical purposes it is one way. For example hydrogen burns with oxygen to make water. However, chemistry can change with temperature and pressure. At ambient temperatures the reverse reaction is absolutely negligible.
What is a reversible reaction?
Updated August 19, 2019. A reversible reaction is a chemical reaction where the reactants form products that, in turn, react together to give the reactants back. Reversible reactions will reach an equilibrium point where the concentrations of the reactants and products will no longer change.
What happens to the energy in a reversible reaction?
In a reversible reaction, reacting molecules in a closed system collide with each other and use the energy to break chemical bonds and form new products. Enough energy is present in the system for the same process to occur with the products. Bonds are broken and new ones formed, that happen to result in the initial reactants.
What is the equilibrium constant of a reversible reaction?
The equilibrium of a reversible reaction depends on the initial concentrations of the reactants and products and the equilibrium constant, K.
When did Berthollet propose the idea of a reversible reaction?
At one time, scientists believed all chemical reactions were irreversible reactions. In 1803 , Berthollet proposed the idea of a reversible reaction after observing the formation of sodium carbonate crystals on the edge of a salt lake in Egypt.
Can weak acids and bases undergo reversible reactions?
Weak acids and bases may undergo reversible reactions. For example, carbonic acid and water react this way:
Do two chemical reactions occur at the same time?
Two chemical reactions occur simultaneously: Reversible reactions do not necessarily occur at the same rate in both directions, but they do lead to an equilibrium condition. If dynamic equilibrium occurs, the product of one reaction is forming at the same rate as it is used up for the reverse reaction.
What happens to energy in reversible reactions?
Energy changes in reversible reactions. If a reaction is exothermic in one direction, it will be endothermic in the other direction. The same amount of energy is transferred in both the forwards and reverse reaction.
What happens when a reversible reaction happens in a closed container?
Dynamic equilibrium. When a reversible reaction happens in a closed container, it reaches a dynamic equilibrium. At equilibrium: the forward and backward reactions are still happening. the forward and backward reactions have the same rate of reaction. the concentrations of all the reacting substances remain constant.
What is the term for the reaction that produces the original reactants?
In some chemical reactions, the products of the reaction can react together to produce the original reactants. These reactions are called reversible reactions. They can be represented in the following way:
Which reaction goes to the right?
the forward reaction is the one that goes to the right. the backward reaction is the one that goes to the left. The reaction mixture may contain reactants and products, and their proportions may be changed by altering the reaction conditions.
Is the forward reaction endothermic or exothermic?
The forward reaction is endothermic and the reverse reaction is exothermic.
Is a chemical reaction reversible?
Chemical reactions are reversible and may reach a dynamic equilibrium. The position of equilibrium of a reversible reaction can be altered by changing the reaction conditions. Part of. Combined Science. The rate and extent of chemical change.
What does negative G mean?
A negative ∆G is indicative of a spontaneous rxn and the larger the value the more likely it will NOT be reversible.
Is strong or weak acid reversible?
All of that is true but it’s easier if you’re talking about acid and bases... you only need to identify if you have a strong or weak acid, strong are complete while weak are reversible. That might be obvious to you but no one mentioned it🤭
Is a reaction reversible or irreversible?
Depending on what level of chem you're taking your teacher might just be looking for you to identify if the product precipitates out of solution (which would be an irreversible reaction). However if we're being thorough all reactions are reversible to some extent, and you need to compare the rate constants of the forward and reverse reactions to determine if the reverse reaction makes a meaningful contribution towards the system at equilibrium.
/GettyImages-481466958-58df20ca3df78c5162560294.jpg)