What is the process of making limestone?
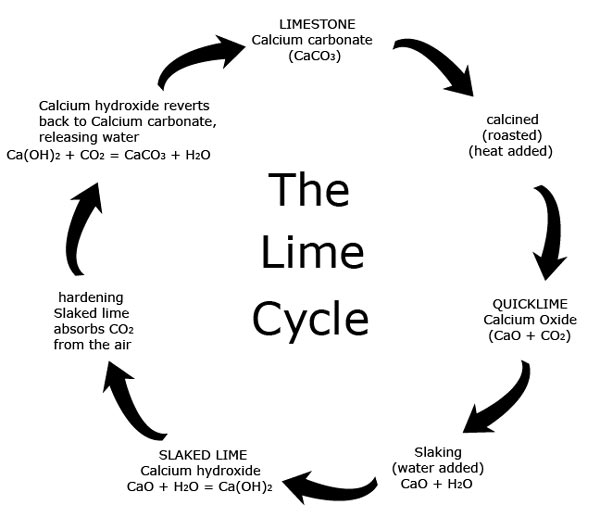
Jun 04, 2020 · Lime is produced through the calcination of limestone (calcium carbonate) in a lime kiln at temperatures at or above 2000 degrees Fahrenheit. The product of calcination of high calcium limestone is "quicklime" or calcium oxide. Quicklime in turn can be reacted with water to produce hydrated lime (calcium hydroxide).
How do you make limestone on alchemy?
In respect to this, how is lime produced from limestone? Lime is produced through the calcination of limestone (calcium carbonate) in a lime kiln at temperatures at or above 2000 degrees Fahrenheit. The product of calcination of high calcium limestone is "quicklime" or calcium oxide. Quicklime in turn can be reacted with water to produce hydrated lime (calcium hydroxide).
How to start mining limestone?
Aug 20, 2012 · In this activity, students view the interactive Calcination – lime from limestone, which shows the industrial processing of limestone into lime, and use the information to complete a matching activity. By the end of this activity, students should be able to: describe the effect of heat on limestone; define the term ‘calcination’
How to make concrete to look like limestone?
20 Why is limestone called limestone? 21 How do they make lime? 22 How is limestone turned into lime? ...

How do you extract limestone from lime?
The limestone is heated as it moves down the kiln toward the lower end. As the preheated limestone moves through the kiln, it is “calcined” into lime. The lime is discharged from the kiln into a cooler where it is used to preheat the combustion air. Lime can either be sold as is or crushed to make hydrated lime.
Is lime and limestone the same?
Limestone is a sedimentary rock that formed millions of years ago as the result of the accumulation of shell, coral, algal, and other ocean debris. Lime is produced when limestone is subjected to extreme heat, changing calcium carbonate to calcium oxide.Jul 17, 2020
Can we make limestone?
Limestone is formed in two ways. It can be formed with the help of living organisms and by evaporation. Ocean-dwelling organisms such as oysters, clams, mussels and coral use calcium carbonate (CaCO3) found in seawater to create their shells and bones.
Is lime made from limestone?
Lime is made by first burning chalk or limestone to form quick lime (calcium oxide) and then slaking the quicklime with water (forming calcium hydroxide).
How do you make limestone?
3:194:31limestone, quicklime and slaked lime | Chemistry | FuseSchool - YouTubeYouTubeStart of suggested clipEnd of suggested clipLine sleek line dissolves in water to form lime water reacts with carbon dioxide to form calciumMoreLine sleek line dissolves in water to form lime water reacts with carbon dioxide to form calcium carbonate. The main component of limestone.
What can replace limestone?
Ashes from lignite or coal, blastfurnace slag, concrete crusher sand, aerated concrete meal and fractions from demolition waste have already been decarbonated and could be used as an alternative to 'virgin' limestone thus avoiding CO 2 emissions during its transformation to lime in the production process.
How is crushed limestone made?
Crushed limestone typically produced by mining limestone or dolomite rock deposits. The process involves breaking of collected rocks down to the specific sizes with the help of different rock crushers/machines. It further screening/filtering into different grit sizes for suitable applications.Jun 22, 2020
How do you make limestone bricks?
Limestone bricks can be crafted through the Crafting skill at level 12 by using a chisel with limestone, granting 6 Crafting experience. Note that you may inadvertently create a rock if you are too heavy-handed with the chisel.
What is limestone powder?
Limestone powder is crushed and ground from natural limestone. Belsazar Hacquet [31] first distinguished limestone from dolomite as sedimentary rock. Limestone is mainly composed of skeletal fragment of organisms.Aug 30, 2018
Why is limestone called limestone?
limestone (n.) late 14c., from lime (n. 1) + stone (n.). So called because it yields lime when burnt. Another name for it, mostly in American English, is limerock.
How do you make lime out of shells?
Burning converts the shells into a material called calcium oxide or quicklime. He collected the fired shells and added water, thereby creating a product called slaked lime or hydrated lime. This process is called "slaking of lime," and changes the chemical composition of the lime yet again.Mar 15, 2018
What is lime from limestone used for?
In construction, the dominant use of lime is in soil stabilization for roads, earthen dams, airfields, and building foundations. Lime can be combined with certain additives to produce other metals and is also a key ingredient in mortar and plaster in lime slurry form.
Tips
If you want to make slaked lime, spray the quicklime with a limited amount of water. It will hiss and crumble, forming calcium hydroxide. If you place the slaked lime in water for a few hours, a clear solution of lime water will form which turns milky as it absorbs carbon dioxide and can thus be used to detect it.
Warnings
Be 100% sure that what you are heating is calcium carbonate, not calcium sulfate. Do not use blackboard chalk for this.
About This Article
This article was co-authored by our trained team of editors and researchers who validated it for accuracy and comprehensiveness. wikiHow's Content Management Team carefully monitors the work from our editorial staff to ensure that each article is backed by trusted research and meets our high quality standards.
Quicklime
Quicklime is made by heating calcium carbonate (limestone, marble, chalk, shells, etc.) to a temperature of around 1000°C for several to “burn” or “calcimine” it.
Hydrated Lime (aka Slaked Lime)
Quicklime is a strong base so it is unstable and can be hazardous. To make it less dangerous, water is added to” slake” it. This is also known as hydrated lime.
What is Limewater?
Limewater comes in two varieties. The first is natural. It’s water that contains a higher-than-normal amount of calcium carbonate or calcium sulfate. The second variety is manmade: “milk of lime.” It’s a solution made from lime, acted upon (or slaked) by water. Lime itself is a solid, white compound of calcium and oxygen.
How to Make the Solution
1. Put 1 teaspoon of calcium hydroxide in a clean glass jar, up to 1 gallon in size. (Limewater is a saturated solution, which means there will be some extra chemical that doesn’t dissolve. A teaspoon will result in a fully saturated solution whether you use a gallon jar or a smaller one.)
