
A battery works on the oxidation and reduction reaction of an electrolyte with metals. When two dissimilar metallic substances, called electrode, are placed in a diluted electrolyte, oxidation and reduction reaction take place in the electrodes respectively depending upon the electron affinity of the metal of the electrodes.
How to activate a battery?
The Process to Activate a Conventional Battery
- The battery must be out of the vehicle and placed on a level surface. ...
- Remove the red sealing cap from the vent elbow. ...
- Using the acid bottle supplied with the battery, place the container upright on a flat surface. ...
- Fill the battery with the electrolyte supplied with the battery. ...
- Fill each battery cell slowly and carefully to the highest level line.
How does a battery generate electrical energy?
How Does a Lithium-ion Battery Work?
- The Basics. A battery is made up of an anode, cathode, separator, electrolyte, and two current collectors (positive and negative).
- Charge/Discharge. While the battery is discharging and providing an electric current, the anode releases lithium ions to the cathode, generating a flow of electrons from one side to the other.
- Energy Density vs. ...
How to identify my battery?
How to create a Battery Report
- Perform a Windows Search for CMD or Command Prompt. ...
- Once open, you’ll see a command line starting with C: and ending with the name of your user account. ...
- If successful, the Command Prompt will tell you a battery life report was saved to your user folder.
- Navigate to the folder listed in the Command Prompt. ...
How to check the battery information in Windows 10?
Part 1 Part 1 of 2: Generating a Report
- Press ⊞ Win + R. This will open the "Run" dialog box.
- Type powershell into the box.
- Press Ctrl +⇧ Shift +↵ Enter at the same time. ...
- Click Yes on the UAC dialog box.
- Type powercfg /batteryreport /output "$home\Documents\battery-report.html" into the PowerShell window.
- Type exit into the PowerShell window. ...
- Open the report. ...
What is a battery?
What are the components of a battery?
What is the purpose of electrolytes?
How does an electrolyte discharge electricity?
Can a battery be reversed?
See 2 more
About this website

How does a battery work explain with an example?
Batteries and similar devices accept, store, and release electricity on demand. Batteries use chemistry, in the form of chemical potential, to store energy, just like many other everyday energy sources. For example, logs store energy in their chemical bonds until burning converts the energy to heat.
How does a battery conduct electricity?
Batteries produce electricity The metal that frees more electrons develops a positive charge, and the other metal develops a negative charge. If an electrical conductor, or wire, connects one end of the battery to the other, electrons flow through the wire to balance the electrical charge.
What is a battery made of and how does it work?
There are three main components of a battery: two terminals made of different chemicals (typically metals), the anode and the cathode; and the electrolyte, which separates these terminals. The electrolyte is a chemical medium that allows the flow of electrical charge between the cathode and anode.
How does a battery work for kids?
0:092:14How do Batteries Work? (With Narration) | Mocomi Kids - YouTubeYouTubeStart of suggested clipEnd of suggested clipWithin the battery the anode builds up an excess of electrons. This causes an electrical differenceMoreWithin the battery the anode builds up an excess of electrons. This causes an electrical difference between the anode.
Why do batteries have a positive and negative side?
Electrons are negatively charged, so they will be attracted to the positive end of a battery and repelled by the negative end. When the battery is hooked up to a device that lets the electrons flow through it, they flow from negative (anode) to positive (cathode) terminal.
How does a 9v battery work?
0:000:57How Does A 9V Battery Work? - Collin's Lab Notes ... - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd that's about the simplest. Answer a nine volt is really 6 very small 1.5 volt cells wired inMoreAnd that's about the simplest. Answer a nine volt is really 6 very small 1.5 volt cells wired in series makes sense it's like they're standing on each other's shoulders with a big trench coat on.
What are the 5 functions of a battery?
Top 5 Functions Of A Car BatteryPower House Of The Car. Power House Of The Car. ... Powers The Ignition System. Powers The Ignition System. ... Without Battery Engine Wont Fire. Without Battery Engine Wont Fire. ... Heart Of The Electrical System. Heart Of The Electrical System. ... Power Serge Regulator.Power Serge Regulator.
What type of energy is stored in a battery?
Chemical energyChemical energy is energy stored in the bonds of atoms and molecules. Batteries, biomass, petroleum, natural gas, and coal are examples of chemical energy.
Which way do electrons flow in a battery?
Electrons flow away from the negative terminal (anode) through the load. Negative OH− ions flow away from the positive terminal (cathode) through the electrolyte. The separator should allow the OH− to flow from the positive terminal to the negative terminal.
How do batteries create energy?
It's the chemical catalyst in the form of a gooey paste or liquid used in between the electrodes to make the battery conductive. It does this by providing the ion transport mechanism between the anode and cathode of the cell. This is where the battery's chemical energy transforms into electrical energy.
How does electrolyte work in a battery?
Electrolyte serves as catalyst to make a battery conductive by promoting the movement of ions from the cathode to the anode on charge and in reverse on discharge. Ions are electrically charged atoms that have lost or gained electrons.
When battery is charged How does electrons move or travel?
Electrons are negatively charged, and so are attracted to the positive end of a battery and repelled by the negative end. So when the battery is hooked up to something that lets the electrons flow through it, they flow from negative to positive.
What is battery in science?
A battery, which is actually an electric cell, is a device that produces electricity from a chemical reaction. Strictly speaking, a battery consists of two or more cells connected in series or parallel, but the term is generally used for a single cell. A cell consists of a negative electrode; an electrolyte, which conducts ions; a separator, ...
What happens when a battery is connected to an external load?
When the cell is connected to an external load, or device to be powered, the negative electrode supplies a current of electrons that flow through the load and are accepted by the positive electrode. When the external load is removed the reaction ceases. A primary battery is one that can convert its chemicals into electricity only once ...
What Is a Nickel Cadmium Battery?
The first NiCd battery was created by Waldemar Jungner of Sweden in 1899 .
Why are nickel batteries being phased out?
Due to worker's safety requirements, processing of cadmium for batteries in the U.S. is already in the process of being phased out.
Why are lithium batteries not available?
They are generally not available to the public because they are less safe than the solid cathode systems. The next step in lithium ion battery technology is believed to be the lithium polymer battery. This battery replaces the liquid electrolyte with either a gelled electrolyte or a true solid electrolyte.
What type of battery is used in watches?
A number of non-rechargeable batteries were first developed with lithium metal as the anode. Commercial coin cells used for today's watch batteries are mostly a lithium chemistry. These systems use a variety of cathode systems that are safe enough for consumer use.
What is the difference between a cadmium battery and a nickel battery?
The cadmium electrode was replaced with a hydrogen gas electrode. This battery is visually much different from the Nickel-Cadmium battery because the cell is a pressure vessel, which must contain over one thousand pounds per square inch (psi) of hydrogen gas.
Why do electrons flow in a battery?
The electrons and ions flow because of the chemical reactions happening inside the battery —usually two of them going on simultaneously.
How long does it take for a battery to release electricity?
Unlike normal electricity, which flows to your home through wires that start off in a power plant, a battery slowly converts chemicals packed inside it into electrical energy, typically released over a period of days, weeks, months, or even years.
What are the main parts of a battery?
There are two electrodes (electrical terminals) and a chemical called an electrolyte in between them. For our convenience and safety, these things are usually packed inside a metal or plastic outer case. There are two more handy electrical terminals, marked with a plus (positive) and minus (negative), on the outside connected to the electrodes that are inside. The difference between a battery and a cell is simply that a battery consists of two or more cells hooked up so their power adds together.
Why don't electrons hop from the negative electrode to the positive electrode?
Now you may be thinking: "Hang on, this doesn't make any sense! Why don't the electrons just take a short cut and hop straight from the negative electrode through the electrolyte to the positive electrode? It turns out that, because of the chemistry of the electrolyte, electrons can't flow through it in this simple way. In fact, so far as the electrons are concerned, the electrolyte is pretty much an insulator: a barrier they cannot cross. Their easiest path to the positive electrode is actually by flowing through the outer circuit.
How are ions formed?
Ions (atoms with too few or too many electrons) are formed from the materials in the electrodes and take part in chemical reactions with the electrolyte. At the same time, electrons march from one terminal to the other through the outer circuit, powering whatever the battery is connected to.
What is the difference between a battery and a cell?
The difference between a battery and a cell is simply that a battery consists of two or more cells hooked up so their power adds together. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts buzzing with activity.
When were flashlight batteries invented?
The cheapest, ordinary, everyday batteries you get for things like flashlights are zinc carbon ones. Disposable zinc-carbon batteries date back to about 1865, when they were invented by French engineer Georges Leclanché; that's why they're sometimes referred to as Leclanché cells.
How does a battery work?
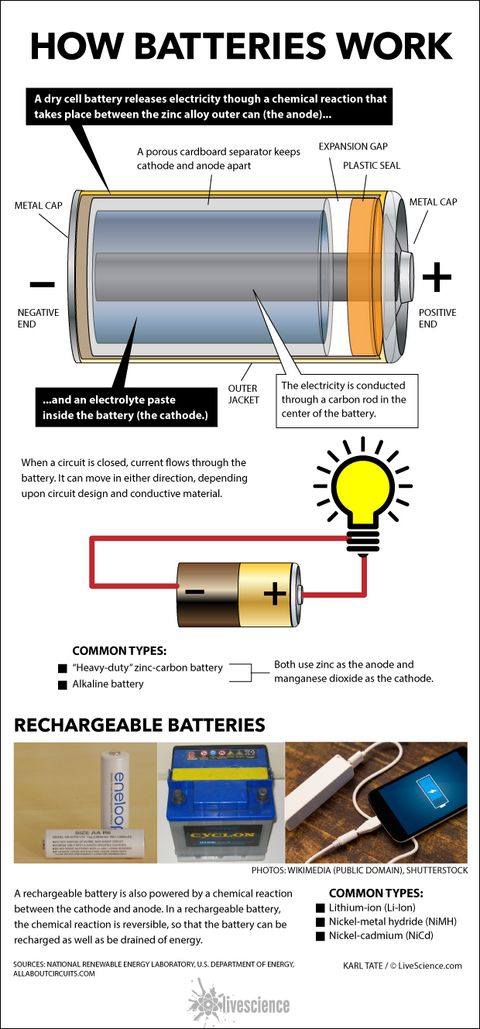
A battery works by completing an circuit within an electrical device. (Image credit: Mrs_ya | Shutterstock) Batteries are everywhere. The modern world is dependent on these portable sources of energy, which are found in everything from mobile devices to hearing aids to cars.
How are batteries powered?
Typical batteries are powered by a chemical reaction. [ See full infographic] (Image credit: by Karl Tate, Infographics Artist)
What is the purpose of a battery separator?
The separator's role is to keep the anode and the cathode separated from each other inside the battery. Without a separator, the two electrodes would come into contact, which would create a short circuit and prevent the battery from working properly, Sastry explained.
Why do you have to throw away batteries?
You have to throw such batteries away, because the electrochemical processes that made the battery produce energy cannot be reversed, Sastry explained. However, the electrochemical processes that occur within secondary, or rechargeable, batteries can be reversed by providing electrical energy to the battery. For example, this happens when you plug your cellphone battery into a charger connected to a power source.
How does a flashlight work?
When you put those batteries into the flashlight and then turn it on, what you're really doing is completing a circuit. The stored chemical energy in the battery converts to electrical energy, which travels out of the battery and into the base of the flashlight's bulb, causing it to light up. Then, the electric current re-enters the battery, but at the opposite end from where it came out originally.
What are the parts of a battery?
Anatomy of a battery. Most batteries contain three basic parts: electrodes, an electrolyte and a separator, according to Ann Marie Sastry, co-founder and CEO of Sakti3, a Michigan-based battery technology startup. RECOMMENDED VIDEOS FOR YOU... There are two electrodes in every battery. Both are made of conductive materials, ...
Why is power density important?
For electric vehicles, power density is important because it tells you how fast the car can accelerate from 0 to 60 mph (97 km/h), Sastry said. Engineers are constantly trying to come up with ways to make batteries smaller without diminishing their power density.
What is the purpose of a battery?
Essentials. A battery is a device that stores chemical energy and converts it to electrical energy. The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. The flow of electrons provides an electric current that can be used to do work.
Why does a battery go flat?
As the battery is used, and the reactions at both electrodes chug along, new chemical products are made. These reaction products can create a kind of resistance that can prevent the reaction from continuing with the same efficiency. When this resistance becomes too great, the reaction slows down. The electron tug-of-war between the cathode and anode also loses its strength and the electrons stop flowing. The battery slowly goes flat.
How does an electrochemical cell get its electricity?
In an electrochemical cell, electrons are produced by a chemical reaction that happens at one electrode (more about electrodes below!) and then they flow over to the other electrode where they are used up. To understand this properly, we need to have a closer look at the cell's components, and how they are put together.
Why don't rechargeable batteries keep working?
Each charge cycle degrades the electrodes just a little bit more, meaning the battery loses performance over time, which is why even rechargeable batteries don’t keep on working forever.
How do charged ions affect the flow of electrons?
To balance the flow of electrons, charged ions also flow through an electrolyte solution that is in contact with both electrodes. Different electrodes and electrolytes produce different chemical reactions that affect how the battery works, how much energy it can store and its voltage. Imagine a world without batteries.
How is electricity produced?
Most simply, electricity is a type of energy produced by the flow of electrons. In an electrochemical cell, electrons are produced by a chemical reaction that happens at one electrode (more about electrodes below!) and then they flow over to the other electrode where they are used up.
What is the purpose of charged ions in an electrolyte solution?
To balance the flow of electrons, charged ions also flow through an electrolyte solution that is in contact with both electrodes.
How many volts does a battery produce?
Because the cells are wired in series, the battery produces approximately 12.6 volts. That voltage leaves the battery through the battery terminals. The battery terminals connect to the battery cables, which, in turn, connect to the vehicle. While supplying electricity to the vehicle, the battery begins to discharge.
What is the purpose of a battery in a car?
The battery in your car serves two primary functions: Cranking the engine to get it started. Providing electrical energy to the vehicle when the alternator does not provide enough (or any) output. The battery also acts as a capacitor to smooth out current ripples and protect the vehicle’s sensitive onboard electronics.
Why is an alternator used in a car?
For this reason, an alternator is used to recharge the battery when the car is running.
What is a flooded battery?
Lead-Acid (aka Flooded) Most 12-volt automotive batteries are of the lead-acid variety. This type of battery is also known as a flooded lead-acid (FLA) battery because it contains a liquid electrolyte. Absorbed Glass Mat (AGM) Another design becoming increasingly common is the AGM battery.
What is the CA rating on a 12V battery?
The CA rating indicates the number of amps a 12-volt battery can deliver for 30 seconds at 32 degrees Farenheight without falling below 7.2 volts. The CA rating is essentially the same thing as another type of rating called the marine cranking amperes.
How many amps can a 12 volt battery supply?
The reserve capacity is the length of time (measured in minutes) that a 12-volt battery can supply 25 amps before dropping below 10.5 volts.
Why is a battery recharging called a storage battery?
Lead-acid batteries are sometimes referred to as “storage batteries” because they are repeatedly discharged and must be recharged by the alternator. Car Battery Ratings.
How do 9V batteries work?
You can increase the voltage within the battery package by doing the series stacking inside it. This is exactly how 9V batteries work - they have 6 small 1.5V cells inside them. If you open a 9V battery you will see these cells. But this is for size convenience rather than power capacity so typically 9V batteries are used for low drain loads where size matters.
Why do the electrons in an alkaline battery move toward the cathode?
As a result, the two electrodes have different charges: The anode becomes negatively charged as electrons are released , and the cathode becomes positively charged as electrons (which are negatively charged) are consumed . This difference in charge causes the electrons to want to move toward the positively charged cathode. However, they don't have a way to get there inside the battery because the separator prevents them from doing so.
What makes a flashlight light up?
All of the parts of the battery work together to make the flashlight light up. The electrodes in the battery contain atoms of certain conducting materials. For instance, in an alkaline battery, the anode is typically made of zinc, and manganese dioxide acts as the cathode. And the electrolyte between and inside those electrodes contains ions. When these ions meet up with the electrodes' atoms, certain electrochemical reactions take place between the ions and the electrodes' atoms.
What is the purpose of a battery separator?
The separator's role is to keep the anode and the cathode separated from each other inside the battery. Without a separator, the two electrodes would come into contact, which would create a short circuit and prevent the battery from working properly.
What is the chemical reaction that occurs in the electrodes?
The series of chemical reactions that occurs in the electrodes are collectively known as oxidation-reduction reactions. In a battery, the cathode is known as the oxidizing agent because it accepts electrons from the anode. The anode is known as the reducing agent, because it loses electrons.
How many electrodes are in a battery?
There are two electrodes in every battery. Both are made of conductive materials, but they serve different roles. One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which is when the battery is being used to power something. The other electrode, known as the anode, connects to the negative end of the battery and is where the electrical current enters (or electrons leave) the battery durin
What is an AA battery?
The AA battery or Mignon battery is a single cell cylindrical dry battery which is commonly used in portable electronic devices. The batteries are usually composed of a single electrochemical cell that is either disposable or rechargeable. 25% of the battery is made with steel, 60% of it is made with some earth elements such as Zinc, Potassium, Manganese or Graphite and the remaining 15% is made up of paper and plastic.
How does a battery system work in daylight?
During daylight, the battery storage system is charged by clean electricity generated by solar.
What is battery energy storage?
Battery energy storage systems are rechargeable battery systems that store energy from solar arrays or the electric grid and provide that energy to a home or business.
What is battery backup?
EMERGENCY BACKUP — Battery energy storage provides the peace of mind that comes with keeping the power on during an outage. Energy storage works with or without solar and is a safe and seamless alternative to small generators, which are one of the main contributors to carbon monoxide poisoning in America.
What are the components of an energy storage system?
Each energy storage unit contains several components: one or more battery modules, onboard sensors, control components, and an inverter. In DC-coupled units, a separate inverter is used. In AC coupled units, the inverter is integrated into the system. These components make energy storage systems more than mere batteries.
What is a swappable battery?
Multiple, swappable battery modules prevent an entire energy storage unit from going down if one battery module fails. The module can be swapped out for another with no downtime.
When is energy discharged from battery storage?
Energy is discharged from the battery storage system during times of high usage, reducing or eliminating costly demand charges.
Do you need batteries for off grid solar?
OFF GRID — Batteries are necessary for a solar-powered off-grid home. Modern battery energy storage systems far exceed the capabilities of the marine lead-acid batteries used by pioneering solar DIYers in decades past. Modern systems are easier to install, easier to configure, more scalable, much cheaper per kWh of storage, and far safer.
What is a battery?
“A battery is a device that is able to store electrical energy in the form of chemical energy, and convert that energy into electricity, ” says Antoine Allanore, a postdoctoral associate at MIT’s Department ...
What are the components of a battery?
There are three main components of a battery: two terminals made of different chemicals (typically metals), the anode and the cathode; and the electrolyte, which separates these terminals. The electrolyte is a chemical medium that allows the flow of electrical charge between the cathode and anode. When a device is connected to a battery — ...
What is the purpose of electrolytes?
The electrolyte is there to put the different chemicals of the anode and cathode into contact with one another, in a way that the chemical potential can equilibrate from one terminal to the other, converting stored chemical energy into useful electrical energy.
How does an electrolyte discharge electricity?
More specifically: during a discharge of electricity, the chemical on the anode releases electrons to the negative terminal and ions in the electrolyte through what’s called an oxidation reaction. Meanwhile, at the positive terminal, the cathode accepts electrons, completing the circuit for the flow of electrons. The electrolyte is there to put the different chemicals of the anode and cathode into contact with one another, in a way that the chemical potential can equilibrate from one terminal to the other, converting stored chemical energy into useful electrical energy. “These two reactions happen simultaneously,” Allanore says. “The ions transport current through the electrolyte while the electrons flow in the external circuit, and that’s what generates an electric current.”
Can a battery be reversed?
These batteries only work in one direction, transforming chemical energy to electrical energy. But in other types of batteries, the reaction can be reversed.
Anatomy of A Battery
How It Works
- To envision how a battery works, picture yourself putting alkaline batteries, like double AAs, into a flashlight. When you put those batteries into the flashlight and then turn it on, what you're really doing is completing a circuit. The stored chemical energy in the battery converts to electrical energy, which travels out of the battery and into t...
Rechargeable vs. Nonrechargeable
- For primary batteries, like those in a flashlight, the reactions that fuel the battery will eventually stop happening, which means that the electrons that provide the battery with its charge will no longer create an electrical current. When this happens, the battery is discharged or "dead,"Sastry said. You have to throw such batteries away, because the electrochemical processes that made …
Battery Lingo
- Although all batteries work in more or less the same way, different kinds of batteries do have different features. Here are a few terms that come up often in any discussion of batteries: Voltage: When it comes to batteries, voltage — also known as nominal cell voltage — describes the amount of electrical force, or pressure, at which free electrons move from the positive end o…