What is the reaction between ethyne and chlorine?
The reaction of ethyne with chlorine can be simple addition across the double bond: However, under the conditions of this experiment, chlorine reacts explosively with ethyne, removing hydrogen from the hydrocarbon and depositing soot, in what is probably a free radical reaction:
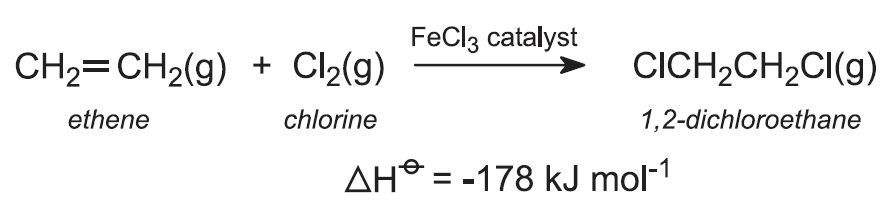
What is the product of the halogenation reaction of ethene and chlorine?
The halogenation reaction takes place in ethene across the double bonds and removes the hydrogen from ethene and two chlorine atoms gets attached in their place; which results in dichloroethane as the product. It is an example for Electrophilic Addition of Halogen. And addition of Cl2 to Alkene is always Trans Addition.
What is the reaction between alkene and ethene?
Answer: The reaction between them is called as halogenation of alkene. Ethene is a hydrocarbon in which double bond is present between carbon-carbon atoms. When chlorine gas is reacted with ethene molecule, it results in the addition of chlorine molecule to the double bond.
What is the reaction between ethene and fluorine?
Ethene reacts explosively with fluorine to give carbon and hydrogen fluoride gas. This isn't a useful reaction, and you aren't likely to need it for exam purposes in the UK at this level (A level or equivalent). Ethene and chlorine or bromine or iodine In each case you get an addition reaction.
See more
What type of reaction occurs between ethene and chlorine?
Addition reaction takes place between ethene and chlorine and it is called halogenations.
Does chlorine react with ethane?
Ethane reacts with chlorine by free radical halogenation in the presence of sunlight when chlorine breaks down to form two chlorine radicals. The chlorine radical reacts with ethane to give ethane radical which reacts with other chlorine to generate to give halogenated product C2Cl6. Was this answer helpful?
What happens when chlorine is added to an alkene?
1. When chlorine is added to an alkene in aqueous (water) solution, another major product besides the dichloro addition product is usually observed. For example, propene reacts with aqueous chlorine to form 1-chloro-2-propanol as the major product.
What is action of chlorine on ethane?
When ethane reacts with chlorine it forms hexachloroethane. C2H6+Cl2→C2H5Cl+HCl.
What are the products formed when chlorine reacts with ethane?
Therefore, the products of the reaction between ethane and chlorine are chloroethane and hydrogen chloride.
What is the action of chlorine on ethene and propene?
Oxidation and Polymerisation of Alkenes.
Does alkene react with chlorine in dark?
It suggests that the hydrocarbon is either alkene or cyclloalkane. It does not react with chlorine in dark.
What products are formed when chlorine reacts with an alkane?
In the presence of light, or at high temperatures, alkanes react with halogens to form alkyl halides. Reaction with chlorine gives an alkyl chloride.
What happens when ethane reacts with HCl?
On reacting ethene with HCl we get chloroethane.
What happen when ethane reacts with halogen?
Ethene and fluorine Ethene reacts explosively with fluorine to give carbon and hydrogen fluoride gas.
What is the action of chlorine on ethane and propane?
Explanation: propane being an alkane reacts with chlorine in a reaction known as substiution reaction forming 1-Chloropropane and hydrogen chloride gas.
Which compounds may result from mixing ethane and chlorine in the presence of sunlight?
When ethane is reacted with chlorine in presence of sunlight or ultra violet light,ethyl chloride is obtained.
What happens when chlorine reacts with ethene?
Explanation : When chlorine reacts with ethene it forms dicholoroethane. It reacts spontaneously to form product. The halogenation reaction takes place in ethene across the double bonds and removes the hydrogen from ethene and two chlorine atoms gets attached in their place; which results in dichloroethane as the product.
Is Cl2 an electrophilic addition?
It is an example for Electrophilic Addition of Halogen. And addition of Cl2 to Alkene is always Trans Addition. That means both cl atoms should add opposite side of double bond and results vicinal 1,2-dichloroethane as a product.
How does chlorine react with ethane?
In this step, one hydrogen atom of ethane is replaced by the chlorine atom. Thus, it is a substitution reaction. The product formed is known as chloroethane.
What is formed when ethene reacts with chlorine?
Ethene and Chlorine or Bromine or Iodine In each case you get an addition reaction. For example, bromine adds to give 1,2-dibromoethane. The reaction with bromine happens at room temperature. Iodine reacts much, much more slowly, but again the chemistry is similar.
How do alkenes react with chlorine?
Explanation: As the non polar chlorine molecule approaches the alkene double bond a dipole is induced in the halogen molecule. The pi bond in the alkene double bond breaks and forms a bond to the delta positive Cl atom, causing the Cl – Cl Bond to break a generating a chloride ion.
Does chlorine react with alkene in dark?
yes , alkenes react with chlorine in the dark. this reaction happens because here π bond present in the alkene .
Does ethane react with chlorine?
When ethane (C2 H6 ) reacts with chlorine (Cl2 ), the main product is C2 H5 Cl; but other products containing Cl, such as C2 H4 Cl2 , are also obtained in small quantities. The formation of these other products reduces the yield of C2 H5 Cl.
Why chlorination of ethane is not possible in dark?
Chlorine is not able to convert into free radicals in the dark, so the reaction doesn’t happen. Hence, presence of sunlight is must for the reaction to occur. Complete step-by-step answer: Methane (CH4) doesn’t react with chlorine in dark because chlorine atoms are pertained in the presence of light.
Why alkene does not react with chlorine in dark?
The process of halogenation of alkanes does not take place in the dark as the initiation of free radicals does not take place in the dark. Thus it must be a cyclo-alkane.
How long does it take for chlorine to explode?
Chlorine gas is evolved. Within a few seconds (or possibly immediately) the two gases will react with explosive ‘pops’, mostly at the surface, giving a yellow flame and black sooty smoke. Intermittent flames will continue for about a minute.
Does chlorine react with ethyne?
However, under the conditions of this experiment, chlorine reacts explosively with ethyne, removing hydrogen from the hydrocarbon and depositing soot, in what is probably a free radical reaction: The intermittent reaction seems to be caused by a buildup of ethyne followed by reaction in which it is all used up.
How does ethane react with chlorine?
Ethane reacts with chlorine by free radical halogenation in the presence of sunlight. In the presence of sunlight, Cl2 breaks down (homolytically) to form two chlorine radicals. This is the initiation step. ... The generated chlorine radical can then react with ethane to give an ethane radical (C2H5*) and HCl.
What happens when ethane radicals react with chlorine?
The ethane radical then reacts with another molecule of chlorine to generate the initial halogenated product and another chlorine radical which can react again. This is the propagation step.
What are the steps of a radical chain reaction?
This is a radical chain reaction. After an initiation step to provide some Cl radicals, there are two chain-propagating steps. (1) chlorine radical abstracts a hydrogen atom from ethane to produce ethyl radical and HCl, and (2) ethyl radical reacts with Cl2 to afford chloroethane and regenerate chlorine atom.
What are the four types of chloroethane?
There is possibility of getting all four types of chloroethane i. e. monochloroethane, dichloroethane, trichloroethane and tetrachloroethane. 2. When ethane is in excess and chlorine is limited :-. When ethane will be in excess then there is one and only one probability of getting chloroethane i. e. monochloroethane.
What happens when chlorine is in excess?
When Chlorine will be in excess then it will give multiple products. There is possibility of getting all four types of chloroethane i. e. monochloroethane, dichloroethane, trichloroethane and tetrachloroethane.
How to get rid of chlorine fumes in air?
Otherwise, I’d suggest getting the fumes out of the air by taking advantage of chlorine’s high solubility in water. Since solutions of chlorine in water are pretty reactive on their own, you’ll want to add something to the spray to neutralize the dissolved chlorine too. Spraying a solution of baking soda in water into the air should take a fair amount of the chlorine out of the air and neutralize it. A solution of urea in water will wo
How many electrons does each chlorine atom get from the two former bonds?
First, Cl-Cl bond will be broken symmetrically. Each chlorine atom will get one electron from two former bonding electron. Species like this one (with single unpaired electron) are called radicals. Formation of radicals in this step is called initiation, and light is needed in this step.
What is the reaction of Krypton and fluorine?from pilgaardelements.com
Krypton will react with fluorine, F2, when cooled to -196 °C (liquid nitrogen) and zapped with an electric discharge or X-rays, form ing krypton(II) fluoride, KrF2[3]. Kr(s)+ F2(s)KrF2(s) This compound decomposes when heating to room temperature.
What is the balancing of the reaction?from pilgaardelements.com
The balancing of the reaction, i.e. the distribution of products depends on the reaction conditions.
Is fluorine explosive?from pilgaardelements.com
Fluorine reacts quickly with hydrogen, forming hydrogen fluoride. The reaction can be explosive under the right conditions [3]:
What is the reaction of chlorine and bromine called?
This is called halogenation . Reactions where the chlorine or bromine are in solution (for example, "bromine water") are slightly more complicated and are treated separately at the end. Simple reactions involving halogens. In each case, we will look at ethene as typical of all of the alkenes.
What happens if you shake an alkene with bromine water?
If you shake an alkene with bromine water (or bubble a gaseous alkene through bromine water), the solution becomes colourless. Alkenes decolourise bromine water. The chemistry of the test. This is complicated by the fact that the major product isn't 1,2-dibromoethane.
What are the complications of alkenes?
There are no complications as far as the basic facts are concerned as the alkenes get bigger. Ethene and fluorine. Ethene reacts explosively with fluorine to give carbon and hydrogen fluoride gas.
Which ion is most likely to be hit by a lone pair on an oxygen of a water?
Instead of the intermediate ion being attacked by a bromide ion, it is much more likely to be hit by a lone pair on an oxygen of a water molecule, followed by loss of a hydrogen ion from the product. Questions to test your understanding.
Can bromine be bubbled?
If you have a gaseous alkene like ethene, you can bubble it through either pure liquid bromine or a solution of bromine in an organic solvent like tetrachloromethane. The reddish-brown bromine is decolourised as it reacts with the alkene.
Is bromine a liquid?
The reddish-brown bromine is decolourised as it reacts with the alkene. A liquid alkene (like cyclohexene) can be shaken with liquid bromine or its solution in tetrachloromethane. Chlorine reacts faster than bromine, but the chemistry is similar. Iodine reacts much, much more slowly, but again the chemistry is similar.
