
What is the difference between fluorite and fluoride?
When remembering the difference between fluoride and fluorite, it is easiest to remember that one is an ingredient of the other. Fluorite is the solid, crystalized form of fluorine, and when broken down, fluoride is a mineral extracted from it. Fluoride in its solid state looks like grains of salt while fluorite looks like a stone or crystal.
What are the effects of iodine fumes?
fever. nausea. not being able to pass urine. thirst, severe. vomiting. Other side effects not listed may also occur in some patients. If you notice any other effects, check with your healthcare professional. Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
Does fluorine and potassium iodide react?
Potassium iodide react with fluorine to produce potassium fluoride and iodine(VII) fluoride. This reaction is proceeds at a temperature of 250°C. Find another reaction. Thermodynamic properties of substances The solubility of the substances Periodic table of elements. Picture of reaction:
Is fluoride good for US?
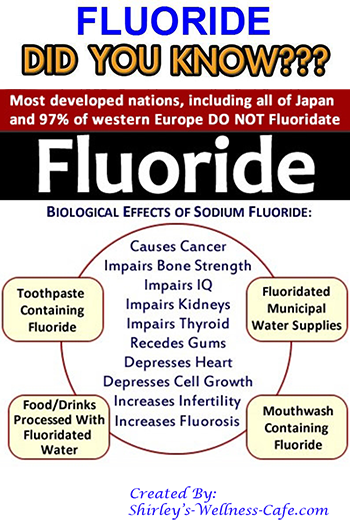
fluoride on the body, but other studies claim fluoride has the capacity to weaken bones and increase the risk of fracture. There is also evidence that fluoride can accumulate in the pineal gland in the brain. Potentially, this could disrupt a range of body processes, including sleep.
Is fluorine or iodine more electronegative?
It is usually measured on the Pauling scale, on which the most electronegative element (fluorine) is given an electronegativity of 4.0. As shown in the figure above, electronegativity decreases from fluorine to iodine; the atoms become less effective at attracting bonding pairs of electrons as they grow larger.
Does fluorine have the highest ionization energy?
Fluorine has the highest ionization energy because it has tendency to gain an electron rather than losing an electron since it has 7 electrons in outermost shell.
What is the Colour of fluorine and iodine?
Relative reactivityfluorineiodineatomic weight18.998126.904colour of elementlight greenish yellowdark violetmelting point (°C)−219.62113.7boiling point (°C)−188.12184.326 more rows
How many energy shells does fluorine have?
List of elements with electrons per shellZElementNo. of electrons/shell6Carbon2, 47Nitrogen2, 58Oxygen2, 69Fluorine2, 766 more rows
Why fluorine has a higher ionisation energy than iodine?
Fluorine has higher ionization energy than iodine because the size of fluorine is smaller than the iodine. This means that the shielding effect is less for fluorine. Therefore, the nucleus attracts more valence electrons in fluorine than in iodine.
How do you know which element has a higher ionization energy?
The ionization energy decreases from top to bottom in groups, and increases from left to right across a period. Thus, helium has the largest first ionization energy, while francium has one of the lowest.
What colour is iodine?
blackIodine is a nonmetallic, nearly black solid at room temperature and has a glittering crystalline appearance.
What is the colour of fluorine?
greenish-yellowSUMMARY Moissan's observation, that fluorine gas is yellow, has been confirmed. At an early stage in their studies, all chemistry students are taught that fluorine is yellow or greenish-yellow in colour [1,2] .
How do the boiling points of halogens change down the group from fluorine to iodine?
The melting points and boiling points of the halogens increase going down group 7. This is because, going down group 7: the molecules become larger. the intermolecular forces become stronger.
Is fluorine a metal?
Fluorine is a non-metal.
What charge does fluorine ion have?
-13.1Computed PropertiesProperty NameProperty ValueReferenceFormal Charge-1Computed by PubChemComplexity0Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)Isotope Atom Count0Computed by PubChemDefined Atom Stereocenter Count0Computed by PubChem14 more rows
What is the ionization energy of fluorine?
The elements of the periodic table sorted by ionization energyIonization EnergyName chemical elementSymbol15,7596ArgonAr17,4228FluorineF21,5645NeonNe24,5874Helium99 more rows
What is the ionization energy of fluorine?
The elements of the periodic table sorted by ionization energyIonization EnergyName chemical elementSymbol15,7596ArgonAr17,4228FluorineF21,5645NeonNe24,5874Helium99 more rows
Does fluorine have higher ionization energy than sodium?
This causes the reactivity of fluorine to other atoms due to it's electron affinity from the unshielded nucleus. It is hard to ionize compared to sodium because of its smaller than sodium, and it's z eff is higher.
Does fluorine have a higher ionization energy than chlorine?
Since the most loosely held electron in fluorine is closer to the nucleus than chlorine, it takes more energy to remove the electron from fluorine than in chlorine.
Does hydrogen or fluorine have a higher first ionization energy?
Now clearly, from the data, we can see that hydrogen has a lower ionization energy when compared to fluorine.
Where did the element iodine come from?
The element Iodine was discovered by Bernard Courtois in year 1811 in France. Iodine derived its name from from French iode (after the Greek ioeides, 'violet')
How many properties can you compare elements on?
Compare elements on more than 90 properties. All the elements of similar categories show a lot of similarities and differences in their chemical, atomic, physical properties and uses. These similarities and dissimilarities should be known while we study periodic table elements.
Why is iodine more polar than flourine?
Iodine has a higher polarizability than flourine because of its sheer size. The bigger the atom, the higher chance that you can have a temporary dipole in that species due to fluctuations in electron position around the nucleus.
Why is fluorine more electronegative than other elements?
Fluorine, compared to other elements is more Electronegative because it requires only one electron to complete an octate and attain stability, and the distance at which valence electrons reside is the minimum as compared with other elements having 7 electrons in the outermost orbit.
Why is the nucleus of an electron cloud weaker?
The weaker attraction of the nucleus on the electrons means that the electron cloud can more easily be distorted (as an analogy, it’s much easier to change the shape of a hot air balloon than a party balloon). It is the distortion of the electron cloud that allows ions to be polarised.
Which element has the highest electronegativity?
Oh, seriously. Every chemistry student in the known universe knows the general trends for electronegativity. Fluorine is the most electronegative element. The electronegativity increases going from left to right across a period. Electronegativity decreases going from top to bottom in a family.
What happens to atomic size when you face the periodic table?
Well, atomic size DECREASES across the Period, a row of the Periodic Table, from left to right as we face the Table, but INCREASES down a Group, a column of the Periodic Table. Of course, the halogens are normally a BIMOLECULE, but here we speak of the ATOM.
Which element is the most electropositive?
The most electropositive element is cesium, which is pyrophoric (will burst into flame in open air) because it wants to lose its lone electron on its outer shell, and because it’s large, that lone electron is far away from the positively-charged nucleus and thus weakly attracted to it, so it cheerfully jumps off.
Is nitroacetic acid stronger than monofluoroacetic acid?
Indeed, it has a stronger deactivating effect in the AES, and, for example, nitroacetic acid is stronger than monofluoroacetic.
Which has a stronger energy color, halogen or halogen?
Once again, the lower atomic number halogen has a weaker energy color (Orange) while the higher atomic number halogen has a stronger energy color (Violet). I'm sure there is some correlation between the electron shells and the reasoning behind the color.
Which element has a stronger energy color?
When you move down to Bromine and Iodine, you start putting in the d subshells and the colors of those halogens are MUCH more intense. Once again, the lower atomic number halogen has a weaker energy color (Orange) while the higher atomic number halogen has a stronger energy color (Violet). I'm sure there is some correlation between the electron shells and the reasoning behind the color. But when looking at the elements with the same kinds of shells (Fluorine/Chlorine, then Bromine/Iodine) you see that the lower atomic number scatters the lower energy color better.
Is iodine the same as violet?
Iodine is the same with violet. When you look at the periodic table, you can kind of make sense of this. Fluorine and chlorine each have s and p orbitals filling up, so they're fairly similar in that regard. Fluorine is a very pale yellow color and chlorine is a pale green.
Do halogens have color?
In regards to the colors of the halogens, it probably does have something to do with the arrangement of the electrons as each of the halogens does have a color to it and that's a unique trait amongst that group. Since the noble gases right after the halogens are colorless, it would make one think that the one electron short of a full shell plays a part in how the halogens absorb/reflect light.
Why can't iodine displace fluoride?
The scientific explanation of the halogen displacement suggests that iodine cannot displace fluoride because it is the heavier halogen. The theory of halogen displacement is the element with the lighter atomic weight can displace the element with the heavier atomic weight, but not the other way around.
Which is better for iodine?
Whole foods are always the better sources of iodine because they contain a complement of nutrients. Seaweeds are the best sources of iodine which include kelp, irish moss, wakame, dulse, arame, hijiki, and nori. Unfortunately the caution against seaweed is the mercury pollution from industry and the radiation from Fukushima entering the Pacific ocean and traveling to the west coast of America. Seaweed absorbs this pollution and radiation, so it is best to get seaweed from the Atlantic.
Which is better, nascent iodine or potassium iodide?
If your choice is short-term supplementation nascent iodine appears to be the better form of supplementation than the use of potassium iodide tablets.
What are the elements that make up halogens?
These halogens are comprised of fluorine, chlorine, bromine, iodine, and astatine . The atomic weight of these halogens are: Fluorine is the lightest element while astatine is the heaviest element.
What are halogens made of?
Halogens are a group of five non-metallic elements that combine with hydrogen to make various forms of salts. These halogens are comprised of fluorine, chlorine, bromine, iodine, and astatine. The atomic weight of these halogens are: 1 Fluorine: 18.99 u 2 Chlorine: 35.45 u 3 Bromine: 79.90 u 4 Iodine: 126.90 u 5 Astatine: 210 u
