ICP-AES, or Inductively Coupled Plasma-Atomic Emission Spectroscopy (also known as ICP-OES, Optical Emission Spectroscopy), is a type of emission spectroscopy that is often used to detect the presence of trace metals in a sample. Through the use of the eponymous Inductively Couple Plasma, an ICP-AES produces excited ions and atoms
What is ICP-AES (ICP-OES)?
ICP-AES, or Inductively Coupled Plasma-Atomic Emission Spectroscopy (also known as ICP-OES, Optical Emission Spectroscopy), is a type of emission spectroscopy that is often used to detect the presence of trace metals in a sample. Through the use of the eponymous Inductively Couple Plasma, an ICP-AES produces excited ions and atoms
What is the ICP principle in spectroscopy?
2. ICP Optical Emission Spectrometry Principle ICP, abbreviation for Inductively Coupled Plasma, is one method of optical emission spectrometry. When plasma energy is given to an analysis sample from outside, the component elements (atoms) are excited.
What is the difference between ICP-OES and ICP-MS?
ICP-OES is often compared to ICP-MS (inductively coupled plasma – mass spectrometry). 57 ICP-MS operates using many of the same principles as ICP-OES, except that the detection of elements from the aerosolized and ionized sample occurs via mass spectral analysis rather than being based on photon emission.
What are the application fields of ICP optical emission spectroscopy?
One major application field of ICP optical emission spectrometry is material analysis. The below example is an analysis of a steel sample. Sample: JSS Standard Steel Sample 150, 0.5g dissolved in 100ml mixed acid of chloric acid and nitric acid.

How does ICP analysis work?
ICP analysis requires the use of liquified sample solutions, so solid samples and biological samples are often digested prior to analysis. Once the sample is liquid, the ICP uses Argon (Ar) carrier gas to aerosolize the sample sending only the smallest droplets through the chamber and into the Argon plasma torch.
Does ICP-AES use a flame?
ICP-OES would be the best choice if the number of samples or the number of elements to be measured is likely to increase. The MP-AES has the lowest running costs and uses no flammable gases.
How does an ICP-OES machine work?
In the ICP-OES the plasma is generated at the end of a quarts torch by a cooled induction coil through which a high frequency alternate current flows. As a consequence, an alternate magnetic field is induced which accelerated electrons into a circular trajectory.
How does AES spectroscopy work?
Atomic emission spectroscopy works by forcing a sample material to a high-energy state using a separate energy source. The wavelengths of light emitted from the sample material's atoms are recorded, and the wavelengths are used to determine the composition of the sample material.
Which gas is used in ICP-AES?
argon gasThrough one of the three thermal mass flow controllers, an argon gas flow enters the nebulizer of the ICP-AES for turning the to-be-analysed sample into a mist. The other two mass flow controllers allow argon to enter the induction-coil surrounded reactor to be turned into a plasma, and for auxiliary purposes.
How does an ICP torch work?
An electric current is passed through the metal coil around the glass torch. The current creates a magnetic field. A spark, discharged into a stream of argon gas creates the plasma. Energy passes from the metal coil around the torch into the argon, sustaining the plasma.
What is the difference between ICP OES and ICP AES?
There is no difference between ICP OES and ICP AES since they are two names for the same technique.
What is the difference between ICP-MS and ICP-AES?
While ICP-AES allows for both trace and major concentrations across a wide range of elements down to part per billion (ppb), ICP-MS provides a lower detection limit down to part per trillion (ppt).
How plasma is generated in ICP?
To generate plasma, first, argon gas is supplied to torch coil, and high frequency electric current is applied to the work coil at the tip of the torch tube. Using the electromagnetic field created in the torch tube by the high frequency current, argon gas is ionized and plasma is generated.
Why is AES surface sensitive?
The technique is inherently surface sensitive because the Auger electrons typically have low kinetic energies (<3kv). The Auger spectra contain information about the concentration and (sometimes) the chemical environment of surface and near-surface atoms.
What is the source of excitation in ICP?
burning flameThe burning flame is referred to as an excitation source because the energy being released from the chemical reaction occurring in the region of the flame is what causes the excitation of the atoms and/or molecules that are being introduced into the flame (the test sample).
What can ICP detect?
It is known and used for its ability to detect metals and several non-metals in liquid samples at very low concentrations. It can detect different isotopes of the same element, which makes it a versatile tool in isotopic labeling.
What is the purpose of ICP-AES?
ICP-AES is an emission spectrophotometric technique, exploiting the fact that excited electrons emit energy at a given wavelength as they return to ground state. The fundamental characteristic of this process is that each element emits energy at specific wavelengths peculiar to its chemical character. Although each element emits energy at multiple wavelengths, in the ICP-AES technique it is most common to select a single wavelength (or a very few) for a given element. The intensity of the energy emitted at the chosen wavelength is proportional to the amount (concentration) of that element in the analyzed sample. Thus, by determining which wavelengths are emitted by a sample and by determining their intensities, the analyst can quantify the elemental composition of the given sample relative to a reference standard.
Can interstitial water be analyzed?
ICP-AES analysis requires a sample to be in solution. Thus, interstitial waters can be analyzed simply, requiri ng only dilution in most cases. Igneous rocks, sedimentary rocks, and sediments, however, must be dissolved.
What equipment is used for ICP?
Equipment for ICP optical emission spectrometry consists of a light source unit, a spectrophotometer, a detector and a data processing unit. There are several types of equipment based on differences in the Spectrophotometer and the detector. The most common type is shown in Figure 1.
What is ICP in spectrometry?
ICP, abbreviation for Inductively Coupled Plasma, is one method of optical emission spectrometry. When plasma energy is given to an analysis sample from outside, the component elements (atoms) are excited. When the excited atoms return to low energy position, emission rays (spectrum rays) are released and the emission rays that correspond to the photon wavelength are measured. The element type is determined based on the position of the photon rays, and the content of each element is determined based on the rays' intensity.#N#To generate plasma, first, argon gas is supplied to torch coil, and high frequency electric current is applied to the work coil at the tip of the torch tube. Using the electromagnetic field created in the torch tube by the high frequency current, argon gas is ionized and plasma is generated. This plasma has high electron density and temperature (10000K) and this energy is used in the excitation-emission of the sample. Solution samples are introduced into the plasma in an atomized state through the narrow tube in the center of the torch tube.
What is the excitation temperature of an argon spectrophotometer?
Compared to atomic absorption spectrophotometers, in which the excitation temperature of air-acetylene flame measures 2000 to 3000 K, the excitation temperature of argon ICP is 5000 to 7000 K , which efficiently excites many elements. Also, using inert gas (argon) makes oxides and nitrides harder to be generated.
Is ICP a multipurpose technique?
ICP optical emission spectrometry is now highly rated as a multipurpose analysis technique and there are over 2,000 units of ICP-OES in use in Japan. It is well regarded as an environmental measurement technique, along with atomic absorption spectrometry and ICP mass spectrometry, and its use is expected to expand even further in the future.
What is an ICP OES?
Inductively coupled plasma optical emission spectroscopy (ICP-OES) and inductively coupled plasma atomic emission spectroscopy (ICP-AES) are used interchangeably in many scientific publications, 26, 27, 28 as both represent the emission of photons from an ionized sample that can be deconvoluted into signals from each of the constituent elements.
What are the strengths of ICP OES?
Key strengths of ICP-OES include the ability to identify the types and ratios of elements in complex samples. For example, ICP-OES has been used effectively to analyze the composition of crude oil, 34 contaminated soil, 35 and heavy metal mixtures, 36 all of which would have been challenging to analyze by other methods. Moreover, the ability to detect multiple elements simultaneously by ICP-OES presents another significant advantage, 37, 38 with researchers reporting situations where ICP-OES has detected up to 19 elements in one analytical procedure. 39 Advances in the ability to aerosolize a broader variety of samples has improved the general applicability of ICP-OES, 40 as have advantages in spectral deconvolution 41 and calibration procedures 17 to facilitate effective detection. Even in the case of radioactive samples, ICP-OES can still be used to determine the elemental composition of the sample, with separate measurements used to determine the degree of radioactivity. 42, 43 Finally, the ease of ICP-OES has allowed it to also be used in chemistry education contexts, 44 with both analytical reagent grade and spectral pure grade solvents, 45 and with relatively high throughput for sample preparation 46 and analysis, 47 highlighting the straightforward usability of the system.
What are the limitations of ICP OES?
Notable limitations of ICP-OES include the fact that samples must be aerosolized. Even though aerosolization procedures have undergone significant advances ( vide supra ), this means that solid and liquid samples cannot be analyzed while they are still in their solid and liquid forms.

Overview
Mechanism
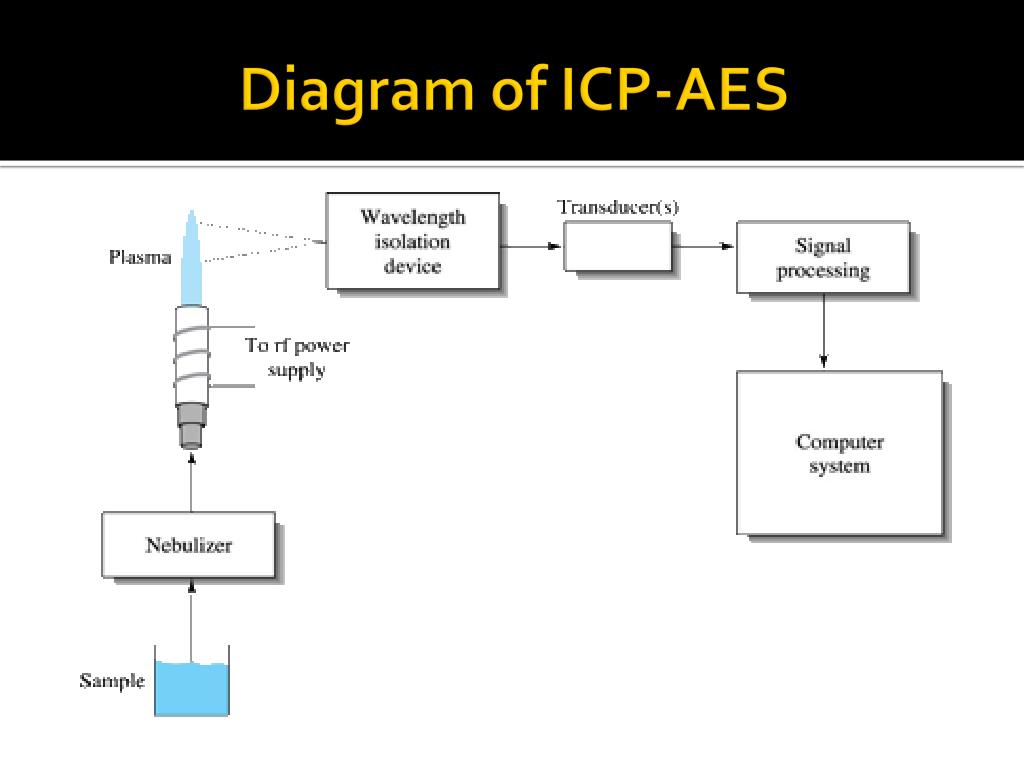
The ICP-AES is composed of two parts: the ICP and the optical spectrometer. The ICP torch consists of 3 concentric quartz glass tubes. The output or "work" coil of the radio frequency (RF) generator surrounds part of this quartz torch. Argon gas is typically used to create the plasma.
The ICPs have two operation modes, called capacitive (E) mode with low plas…
History
The first published attempt to use plasma emissions as a source for spectroscopic analysis were in 1956 by Eugen Bădărău. In 1964 Stanley Greenfield working at Albright & Wilson was the first to use ICP for non experimental analysis. The first commercial machine was produced by KONTRON in 1975.
Applications
Examples of the application of ICP-AES include the determination of metals in wine, arsenic in food, and trace elements bound to proteins.
ICP-OES is widely used in minerals processing to provide the data on grades of various streams, for the construction of mass balances.
In 2008, the technique was used at Liverpool University to demonstrate that a Chi Rho amulet foun…
See also
• Atomic emission spectroscopy
• Atomic absorption spectroscopy
• Inductively coupled plasma mass spectrometry
• Ashing
External links
• Inductively Coupled Plasma/Optical Emission Spectrometry in Encyclopedia of Analytical Chemistry
• Inductively-Coupled Plasma (ICP) Excitation Source [User name and password required]