How does increase in surface area affect the rate of evaporation?
The surface area affects evaporation because if more area is exposed to air, allowing water molecules acquire more heat energy from the surroundings. Due to the increased heat energy (kinetic energy), there is more rapid movement of the water molecules which helps them to overcome the force of attraction and evaporate.
How does the surface area affect air resistance?
How does surface area affect air resistance? The greater the cross-sectional area of an object, the greater the amount of air resistance it encounters since it collides with more air molecules. When a falling object has a large mass, it weighs more and will encounter a greater downward force of gravity.
Does the surface area affect the rate of evaporation?
Molecules contained in a liquid evaporate from the surface area. This means that the larger the surface area, the faster the rate of evaporation. Test this by putting water into two different containers. This means all the other factors that affect the rate of evaporation are identical.
Does surface area affect the rate of dissolving?
The surface area does not affect how much of a solute will be dissolved, but it is a factor in how quickly or slowly the substance will dissolve. Which of the following factors will cause a solid to dissolve faster in a liquid? Increasing the surface area of the solute will increase the rate of dissolving.
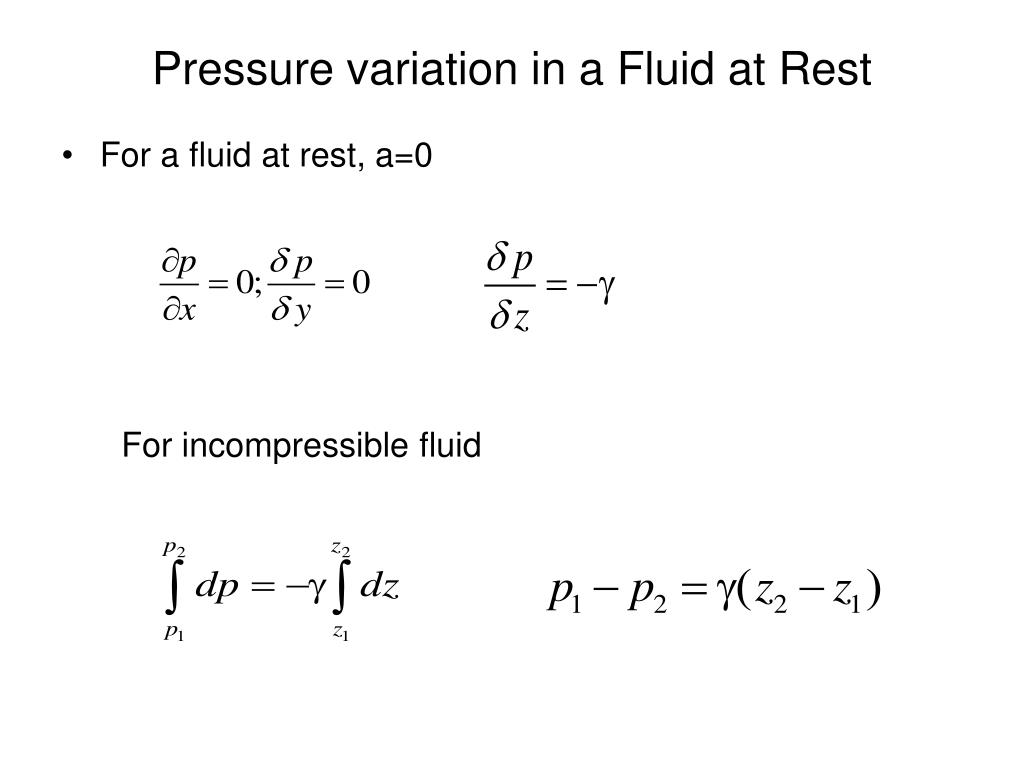
How is vapour pressure related to surface area?
Vapor pressure depends on the composition of the liquid and the temperature. Vapor pressure neither depend on the total surface area of the liquid, nor on the volume of the liquid in the container, nor on the volume of container itself, just as long as some liquid remain when equilibrium is reached.
How surface area and temperature affect the vapor pressure?
therefore, the rates of both evaporation and condensation are increased to the same extent. so the vapour pressure remains the same at constant temperature and is not affected by the surface area of the liquid.
What happens to vapor pressure as surface area increases?
Nothing happens to the vapour pressure as the surface area increases.
What factors affect the vapor pressure of a liquid?
Three common factors that influence vapor press are surface area, intermolecular forces and temperature. The vapor pressure of a molecule differs at different temperatures.
Why surface area has no effect on vapour pressure?
Vapor pressure does not depend on surface area because it is derived from the thermodynamic equilibrium between the liquid and gas phases of a substance. The closer the vapor pressure is to atmospheric pressure, the closer that substance is to boiling.
What happens to the vapor pressure of a substance when its surface area is decreased?
An decrease in surface area decreases both the rate of vaporization and the rate of condensation—the effects exactly cancel, and the vapor pressure does not change.)
What causes higher vapor pressure?
As the temperature of liquid increases, the kinetic energy of its molecules also increases. As the kinetic energy of the molecules increases, the number of molecules transitioning into a vapor also increases, thereby increasing the vapor pressure.
How does surface area affect evaporation?
Evaporation increases with an increase in the surface area If the surface area is increased, then the amount is of liquid that is exposed to air is larger. More molecules can escape with a wider surface area. For e.g. We spread out clothes to dry. We do that because that speeds up the process of vaporization.
How is the rate of evaporation of a liquid affected by the surface area of a liquid exposed to air?
The greater the surface area of a liquid exposed to air, the greater the rate of evaporation.
Which of the following factors affect the vapor pressure of a liquid quizlet?
Intermolecular forces and temperature are the only factors that affect vapor pressure. For a given liquid, temperature is the only factor.
Which of the following factors would not affect the vapor pressure of a liquid?
Because the rate of evaporation and the rate of condensation change by the same degree, the vapor pressure of the liquid does not change. The factor which does not affect the vapor pressure of the liquid is, therefore, the volume of the liquid, or answer choice (B).
Which factor do not affect the vapour pressure of a liquid at equilibrium?
If the volume is increased then some liquids evaporate to form the vapour. Hence, the change in volume will not affect the vapour pressure, so the correct option is B. Note: The other factors that affect vapour pressure are humidity and surface area.
How does temperature affect vapour pressure of a liquid?
As the temperature of a liquid or solid increases its vapor pressure also increases. Conversely, vapor pressure decreases as the temperature decreases.
How does surface area affect evaporation?
Evaporation increases with an increase in the surface area If the surface area is increased, then the amount is of liquid that is exposed to air is larger. More molecules can escape with a wider surface area. For e.g. We spread out clothes to dry. We do that because that speeds up the process of vaporization.
How is surface area related to the rate of evaporation of a liquid and vapor pressure?
As surface area increases, rate of evaporation also increases. This is because evaporation occurs among the molecules on the surface, not in the interior. This means; increasing the surface area will increase the chance of more molecules to evaporate.
How is the rate of evaporation of a liquid affected by the surface area of a liquid exposed to air?
The greater the surface area of a liquid exposed to air, the greater the rate of evaporation.
Why does vapour pressure not depend on volume?
The vapour pressure doesn’t depend on the volume of liquid because the vapour pressure is determined by the properties of the liquid’s surface.
What is vapor pressure?
The vapor pressure is the pressure of the vapor in equilibrium with the liquid at constant temperature. It does not depend on the amount of liquid.
What happens when you half fill a container with a liquid?
What immediately happens? Some of the atoms (or molecules) of liquid break free of the surface tension and begin to populate a gaseous vapor that fills the rest of the container. After a while, the number of gas vapor atoms builds up until equilibrium is reached where the rate of atoms going back into the liquid is exactly matched by the rate of atoms breaking free of the liquid surface. That amount of gas is the vapor pressure of the liquid at that temperature.
How does the rate of evaporation and condensation of a liquid change at equilibrium?
Both are proportional to the surface area of the liquid. If you increase the surface area by increasing the size of the container , you increase the rate of evaporation and condensation by the same amount. The ratio of rates stays the same, so the equilibrium pressure stays the same.
Why do gases come out of liquids?
You need to remember that gases are coming out of the liquid because some of the molecules in that liquid have enough energy to escape the attractions that keep them in liquid form. Whether you’re in a closed container or open container, these gases are still forming and they are forming at a specific rate (speed) that is dependent on the temperature.
What is the ideal gas equation?
The ideal gas equation is PV = nrt. What are the units for pressure?
Does condensation increase surface pressure?
as the rate of evaporation and condensation both increases the surface so the vapour pressure remains the same. for detail see video.
When does surface area evaporate?
When the surface area of a liquid is wide, the more molecules will be exposed on the surface ready to evaporate. But these molecules will evaporate (transition to gas), if and only if they have sufficient energy to overcome the attractive forces between them. A way to think about surface area in this sense is to picture water in an open swimming pool and water in an opened tiny bottle.
When the temperature of a liquid is held constant, what is the vapor pressure?
When the temperature of a liquid is held constant, its vapor pressure is the pressure exerted by its own vapor on its liquid phase when this vapor is in equilibrium with its own liquid phase. See the picture below:
How does temperature affect the rate at which water evaporates?
As temperature increase, the kinetic energy of water molecules also increases. This increase in kinetic energy enables water molecules break the attractive forces that hold them together in the liquid state. As more and more water molecules break these attractive forces, the more they gain the freedom to move and spread, transitioning into steam (gas).
How does strength of attractive forces affect the rate at which water evaporates?
When the attractive forces between water molecules is stronger, the rate at which water turns into steam (evaporates) is slower . This is because water molecules must absorb enough energy to increase their kinetic energy so that they can move away from each other. This moving away from each other is what we call breaking the attractive forces between them. But since this energy is hard to come by, only fewer water molecules will be successful at increasing their kinetic to such a point that it is sufficient to overcome these attractive forces.
What happens when water molecules lose energy?
As they continue to do this, some molecules will lose energy and become less energetic, while others will absorb energy and become more energetic. The slower and less energetic ones will become attracted by or stick to other water molecules in the liquid phase, turning them into liquid water as a result. This process of water vapor turning ...
Why does rubbing alcohol evaporate faster than water?
This happens because the attractive forces between molecules in rubbing alcohol is weaker than the attractive forces between water molecules. These weaker attractive forces in rubbing alcohol causes it to evaporate faster than water. To learn about why salt water has a lower vapor pressure than pure water, click here.
How does water turn into gas?
As they move away, the distance between water molecules increase and so these water molecules transition into gas. This process of water turning into water vapor is usually called evaporation.
What is the vapor pressure of a liquid?
The Vapour pressure of a liquid depends upon the nature of the liquid. A liquid having low boiling points will show high vapor pressure white liquid with high boiling points will have a w vapor pressure.
Which forces have a higher vapor pressure?
Liquids having stronger intermolecular forces have low vapor pressure while liquids with weaker intermolecular forces have high vapor pressures. Hence there is a very close relationship between the strength of intermolecular attractive forces and vapors pressure.
What is the pressure exerted by the vapors in equilibrium with the liquid at a given temperature?
The pressure exerted by the vapors in equilibrium with the liquid at a given temperature is called vapor pressure.
What is the state of equilibrium when the temperature is constant?
These vapors are recaptured by the liquid and the process is called condensation. At constant temperature the rate of both the processes becomes equal and this is the state of dynamic equilibrium.
What is the state of equilibrium between condensation and evaporation?
Both the process of condensation and evaporation continue till the rates of both the processes become equal. This state is called dynamic equilibrium.
What happens when a liquid collides with a container?
These molecules collide with the walls of the container as well as with the surface of the liquid. In this way, they lose some of their kinetic energy and there is a chance that these molecules are recaptured by the liquid surface. This process is known as condensation.
What happens to high energy molecules in containers?
High energy molecules in containers leave the surface of the enclosed liquid and gather above the surface in the empty space in the form of vapors.
