
What is the central organ for regulation of metabolism?
The hypothalamus has a central function in the neural networks that regulate glucose metabolism. The arcuate nucleus of the hypothalamus (ARH) is located at the bottom of the third ventricle and two neuronal populations in the ARH are considered to be critical for the brain’s regulation of energy balance and glucose metabolism.
What occurs when glycogen metabolism is stimulated by insulin?
One of the important effects of insulin on intracellular metabolism is its ability to stimulate the synthesis of glycogen in muscle and liver. It does this by promoting a net decrease in the extent of phosphorylation of glycogen synthase, the rate-limiting enzyme in the pathway of glycogen synthesis, which increases its activity.
Does glycogen require insulin to be used?
Glycogen is a form of stored ... it’s usually because they have diabetes and require insulin to help manage their blood glucose levels. ... While the vast majority of people who use insulin do ...
Do proteins help regulate metabolism?
The act of digesting protein therefore boosts your metabolism more than other nutrients. Diets high in protein may also make you feel full faster, helping you to eat less. Although adding protein to your diet may help boost your metabolism, higher protein intake alone will not cause you to burn significantly more calories.
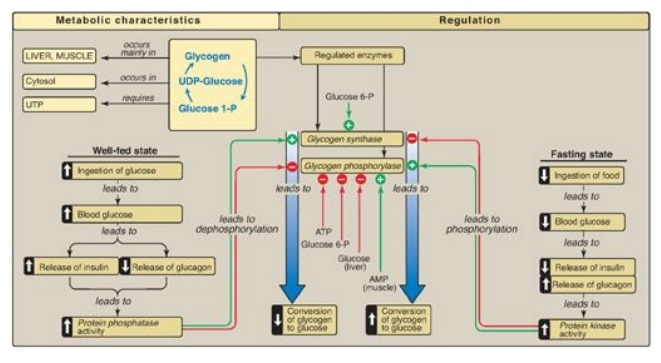
What regulates glycogen metabolism?
The major factor that controls glycogen metabolism in the liver is the concentration of phorphorylase alpha. Indeed, this enzyme catalyzes the limiting step of glycogen breakdown and, by controlling the activity of synthetase phosphatase, also regulates glycogen synthesis.
How does the body regulate glycogen?
The liver is the central organ for regulation of glucose and glycogen and acts as the primary distributor of nutrients through the blood to other tissues. When in a fasted state, the liver breaks down glycogen stores, producing glucose for other tissues.
How is glycogen metabolism regulated by glucagon?
In addition to promoting glycogenolysis, glucagon inhibits glycogen synthesis by regulating glycogen synthase in the liver (Fig. 2). Glycogen synthase plays a key role in glycogen synthesis by catalyzing the transfer of glucosyl residue from UDP-glucose to a nonreducing end of the branched glycogen molecule.
How is glycogen metabolism regulated by a insulin?
Insulin promotes dephosphorylation and activation of glycogen synthase (GS) by inactivating glycogen synthase kinase (GSK) 3 through phosphorylation. Insulin also promotes glucose uptake and glucose 6-phosphate (G-6-P) production, which allosterically activates GS.
How do you increase glycogen metabolism?
Repeated intake of 1.0–1.2 g carbohydrate/kg BW/hour after exercise takes advantage of these metabolic circumstances to stimulate high rates of glycogen synthesis (10–11 mmol/kg wet weight/h).
Why do glycogen metabolism takes place?
In muscle, the role of glycogen is to provide glucose-6-phosphate in response to the ATP for the muscle contractions. Whereas in liver, glycogen provides free glucose for maintaining the blood glucose level.
What is glycogen metabolism called?
Molecular. Glycogenesis. Glycogenesis or glycogen synthesis is a multi-step process that begins with converting glucose to glucose-6-phosphate via hexokinase or the liver isoform of hexokinase known as glucokinase. This process is an essential step as the addition of a phosphate group traps glucose within the cell.
How is glycogen metabolism regulated by insulin glucagon and epinephrine?
Insulin stimulates storage of lipids, proteins, and glycogen. Glycogen synthase is stimulated by glucose-6-phosphate, insulin, and cortisol. It is inhibited by epinephrine and glucagon.
How is glycogen synthesis regulated?
Glycogen synthesis is primarily regulated by modulating the activity of glycogen synthase. This enzyme exists in two forms, dephosphorylated (active or a) and phosphorylated (inactive or b). It is regulated by covalent modification, in an inverse direction to that of glycogen phosphorylase.
What enzyme breaks down glycogen?
In the lysosomes, the breakdown of glycogen is accomplished by the lysosomal enzyme acid α-glucosidase or acid maltase (Fig. 4).
What effect does insulin have on glycogen metabolism?
Insulin inhibits gluconeogenesis and glycogenolysis, stimulates glycolysis and glycogenesis, stimulates uptake and incorporation of amino acids into protein, inhibits protein degradation, stimulates lipogenesis, and suppress lipolysis (Bassett, 1975. (1975).
Do you need insulin to store glycogen?
Insulin is a gatekeeper that allows blood glucose molecules to be used by the cell. When blood glucose levels are low, glucagon is the primary form of hormone that causes the body to release stored glycogen, or energy. For the body to make energy, insulin must allow glucose to enter the cell.
How is glycogen synthesis regulated?
Glycogen synthesis is primarily regulated by modulating the activity of glycogen synthase. This enzyme exists in two forms, dephosphorylated (active or a) and phosphorylated (inactive or b). It is regulated by covalent modification, in an inverse direction to that of glycogen phosphorylase.
How is glycogenolysis regulated?
Glycogenolysis is the biochemical pathway in which glycogen breaks down into glucose-1-phosphate and glucose. The reaction takes place in the hepatocytes and the myocytes. The process is under the regulation of two key enzymes: phosphorylase kinase and glycogen phosphorylase.
Does the body burn glycogen before fat?
Your muscles first burn through stored glycogen for energy. “After about 30 to 60 minutes of aerobic exercise, your body starts burning mainly fat,” says Dr. Burguera. (If you're exercising moderately, this takes about an hour.)
How are glycogen synthesis and breakdown regulated?
Allosteric regulation of glycogen synthesis and breakdown is done by regulation of enzymes glycogen synthase and glycogen phosphorylase. Hormonal regulation of glycogen synthesis and breakdown is done by hormones insulin and glucagon.
How is glycogen metabolism regulated in the liver?
Glycogen metabolism in liver is regulated by phosphorylation and dephosphorylation of regulatory and metabolic enzymes. Control of the phosphorylation state is mediated by Ca2+, cAMP, cytosolic glucose concentration, and perhaps, in the case of insulin, by another mechanism.
How does glycogen metabolism work?
Regulation of glycogen metabolism involves a number of factors coordinating the glycogen synthetic rate with the physiology of the cell. Genetic regulation of the glycogen biosynthesis pathway by cAMP and ppGpp allows E. coli to adjust its metabolic capacity for converting available carbon substrate into glycogen in response to the availability of carbon, energy, or amino acids, respectively. When cells are rapidly mutiplying, the levels of the enzymes are repressed, and although the energy and carbon are available for glycogen synthesis, the glycogen synthesis rate is low. Upon nutrient deficiency, synthesis of ADP-Glc PPase and glycogen synthase are induced, and the capacity for glycogen synthesis is greater. The level of glycogen that is ultimately accumulated will be dependent upon carbon availability and is subject to allosteric regulation of the ADP-Glc PPase activity.
What is glycogen storage disease?
One disease, glycogen storage disease 0, is associated with mutations that impair liver glycogen synthesis and patients are prone to low blood glucose (hypoglycemia). Lafora disease, a progressive myoclonus epilepsy, is characterized by the formation of Lafora bodies in neurons and other tissues.
What is the role of yeast in food?
Saccharomyces cerevisiae (baker's yeast) is an important microorganism used in food technology which mobilizes glycogen after glucose is exhausted from the medium and in the budding process as carbon and energy sources. Recently, it was found that glycogen plays another important role when S. cerevisiae is submitted to stress conditions in the medium of glucose. The effect of stress is to stimulate the recycling of glycogen instead of promoting either its accumulation or its degradation. It is important to note that, under stress, cell growth is arrested, thus there are ATP imbalances in the upper and lower part of the glycolytic pathway. Like a ‘turbo’ design model, glucose oxidation by glycolysis leads to overproduction of ATP, glucose 6-phsphate, phosphate depletion, and cell death. The solution to this cellular problem is possible because stress induces transcriptional activation and the relative enzymes for glycogen metabolism are under this control. Moreover, as a result, both synthesis and degradation enzymes are expressed to the same extent. Thus, it has been suggested that glycogen turnover may function as a glycolytic safety valve to avoid substrate-accelerated death and also that glycogen in these cells confers survival advantages for cell proliferation and stress resistance. In this manner, the glycogen level or glycogen used as a futile cycle contributes to the fitness of the yeast in different environments and under industrial processes. In brewery fermentation yeast is added at the beginning of each batch for eight or more cycles. Before each cycle it is replaced in proliferation conditions and must break down its glycogen for sterol and fatty acids biosynthesis. Glycogen is the sole carbon source used in membrane component biosynthesis. Naturally, the glycogen content at the beginning of the brewery fermentation has a direct impact on yeast vitality (metabolic activity) and a significant improvement in brewer quality.
Why is glucose an effective regulator?
Glucose can be an effective regulator because its concentration in the hepatocyte varies with its concentration in the blood. The postprandial rise in blood glucose contributes to the inhibition of hepatic glycogenolysis and to the activation of glycogen synthesis.
What chapter is glycogen metabolism?
Glucose and glycogen metabolism and the operation of the citric acid cycle are discussed in Chapters 13 and 25. (The disorders of carbohydrate metabolism associated with muscle disease are noted in Chapters 32 and 33 .)
Why do lafora bodies have a large amount of poorly branched glycogen?
Lafora bodies contain large amounts of poorly branched glycogen and may be caused by impaired metabolism of the small amount of phosphate found in glycogen. Table 1. Selected glycogen storage diseases (GSD) GSD type. Name.
Glycogenolysis
Release Release of a virus from the host cell following virus assembly and maturation. Egress can occur by host cell lysis, exocytosis, or budding through the plasma membrane. Virology: Overview
Regulation of Glycogen Metabolism
Glycogen metabolism is controlled by allosteric (metabolites indicate the cellular energy state and help in modulation) and hormonal regulation .
Clinical Relevance
Glycogen storage disease: a group of inherited disorders characterized by abnormalities in glycogen metabolism, resulting in an abnormal accumulation of glycogen in the tissues.
