
Spectroscopy
- Electron Energy Levels. Light can be thought of as both a wave and a particle. ...
- Spectrographs. Astronomers use spectrographs attached to telescopes to view the spectral lines of stars and other celestial objects.
- Applications of Spectroscopy. ...
Why do astronomers use spectrographs?
Spectrographs Most large telescopes today have spectrographs. A spectrograph (spek truh graf) breaks the light from an object into colors and photographs the resulting spectrum. Astronomers use spectrographs to get information about stars, including their chemical compositions and temperatures.
What is the purpose of spectroscopy in astronomy?
Why is Spectroscopy Important?
- Astronomers determine the temperature, density, mass, and motion of an object in the space or coming towards Earth through spectroscopy.
- Doppler Effect in a spectral line tells us the speed of the object coming towards Earth.
- It transforms light into a spectrum using a prism that can be observed through a telescope.
How and why do astronomers use infrared?
Infrared astronomy is a natural tool to use in studying star formation because infrared light penetrates the surrounding dust and be— cause protostars are expected to emit infrared light. … Because of these facts, infrared observations have been the primary means used to search for stars in the process of formation.
What are the disadvantages of spectroscopy?
Disadvantages of Raman spectroscopy
- Raman spectroscopy is very sensitive
- Quite costly equipment.
- Metal or alloy can not be used.
- Difficult to measure low concentrate on samples
- Sample heating through the laser radiation can destroy sample.
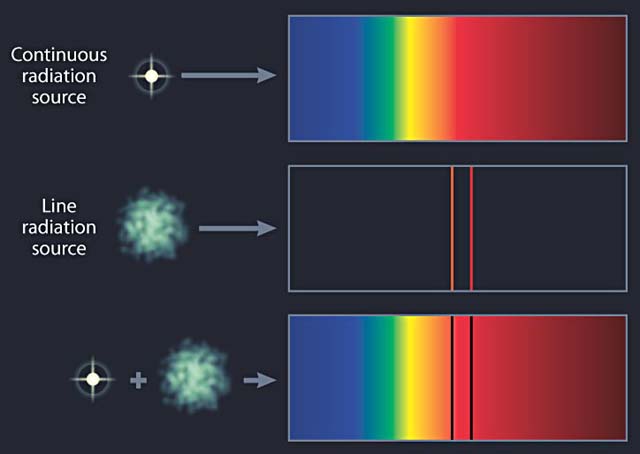
Why is spectroscopy a useful tool in astronomy?
1 Answer. Spectroscopy helps astronomers to determine the composition, temperature, density, and motion of an object. Infrared spectroscopy helps to identify the atoms and molecules in the object.
What is spectroscopy Why is spectroscopy important for modern astronomy?
Through spectroscopy, astronomers can examine different wavelengths of radiation coming from very distant objects in the Universe, from single stars to massive galaxies, and determine their chemical composition and age, track their motion, and much more.
For what reason do astronomers use spectroscopy quizlet?
Spectroscopy is the observational method used by astronomer to infer the nature of matter by the way it emits and absorbs radiation. Spectroscopy can be used to infer the temperature and composition of a star through studying the patterns of dark absorption lines found in the spectrum of the star.
What does spectroscopy reveal about our universe?
A spectrum allows astronomers to determine many things about the object being viewed, such as how far away it is, its chemical makeup, age, formation history, temperature and more.
What is the importance of spectroscopy?
Spectroscopy helps bridge that knowledge gap. It is a method of understanding molecules by measuring the interaction of light and matter. By analyzing the amount of light absorbed or emitted by a sample, we can determine the sample's components, characteristics and volume.
What is spectroscopy quizlet astronomy?
Spectroscopy. the study of the way in which atoms absorb and emit electromagnetic radiation; this is the study of light.
What is spectroscopy and how does it work?
Spectroscopy is the study of the absorption and emission of light and other radiation by matter. It involves the splitting of light (or more precisely electromagnetic radiation) into its constituent wavelengths (a spectrum), which is done in much the same way as a prism splits light into a rainbow of colours.
How do astronomers use spectroscopy to determine elements in stars?
Each element absorbs light at specific wavelengths unique to that atom. When astronomers look at an object's spectrum, they can determine its composition based on these wavelengths. The most common method astronomers use to determine the composition of stars, planets, and other objects is spectroscopy.
What is spectrometer in astronomy?
A spectrometer is a device that forms a spectrum, often utilizing the phenomenon of dispersion. The light from an astronomical source can consist of a continuous spectrum, an emission (bright line) spectrum, or an absorption (dark line) spectrum. Because each element leaves its spectral signature in the pattern of lines we observe, spectral analyses reveal the composition of the Sun and stars.
What is the key to modern astronomy?
Such analysis of spectra is the key to modern astronomy. Only in this way can we “sample” the stars, which are too far away for us to visit. Encoded in the electromagnetic radiation from celestial objects is clear information about the chemical makeup of these objects. Only by understanding what the stars were made of could astronomers begin to form theories about what made them shine and how they evolved.
Why do we see dark lines in the solar spectrum?
The dark lines in the solar spectrum thus give evidence of certain chemical elements between us and the Sun absorbing those wavelengths of sunlight. Because the space between us and the Sun is pretty empty, astronomers realized that the atoms doing the absorbing must be in a thin atmosphere of cooler gas around the Sun. This outer atmosphere is not all that different from the rest of the Sun, just thinner and cooler. Thus, we can use what we learn about its composition as an indicator of what the whole Sun is made of. Similarly, we can use the presence of absorption and emission lines to analyze the composition of other stars and clouds of gas in space.
What is the purpose of electromagnetic radiation?
Electromagnetic radiation carries a lot of information about the nature of stars and other astronomical objects. To extract this information, however, astronomers must be able to study the amounts of energy we receive at different wavelengths of light in fine detail. Let’s examine how we can do this and what we can learn.
What is an emission spectrum?
emission spectrum: a series or pattern of bright lines superimposed on a continuous spectrum. spectrometer: an instrument for obtaining a spectrum; in astronomy, usually attached to a telescope to record the spectrum of a star, galaxy, or other astronomical object. CC licensed content, Shared previously. Astronomy.
How does light affect telescopes?
Light exhibits certain behaviors that are important to the design of telescopes and other instruments. For example, light can be reflected from a surface. If the surface is smooth and shiny, as with a mirror, the direction of the reflected light beam can be calculated accurately from knowledge of the shape of the reflecting surface. Light is also bent, or refracted, when it passes from one kind of transparent material into another—say, from the air into a glass lens.
Who built the spectrometer?
In 1802, however, William Wollaston built an improved spectrometer that included a lens to focus the Sun’s spectrum on a screen. With this device, Wollaston saw that the colors were not spread out uniformly, but instead, some ranges of color were missing, appearing as dark bands in the solar spectrum.
Introduction
The study of spectral lines emitted by objects is spectroscopy. Objects emit radiation at different wavelengths and an instrument called a spectroscope analyzes it. Almost all objects will give off and absorb radiation. We know that radiation is just energy.
Spectral Lines From Radiation
When you analyze spectral lines, you are studying a spectrum of light. It is the division of the radiation’s component wavelengths. This means the light has been divided up. Every object gives off multiple forms of radiation. These will be at different wavelengths.
Emission Line Spectra
CapBy The original uploader was Dragons flight at English Wikipedia. - Transferred from en.wikipedia to Commons by Pieter Kuiper using CommonsHelper., CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=9690052tion
Decomposing Starlight
The last couple hundred years the field of spectroscopy has developed quickly and so scientists had several ideas on how to read starlight. One of the most important things that was learned was that the lines on a spectrum corresponded to individual elements. So developing a spectrum for an object meant we could discern its composition.
Atoms and Radiation
Atoms are the basic units from which everything is created. Even at their small size radiation affects them. Atoms absorb radiation which changes the atom. Historically, the electrons in an associated atom were thought to be on precise orbits, kind of like planets.
Electrons
Electrons are only found in atoms having a certain transfer. This goes whether they are absorbing or transmitting energy. So the radiation involved in these transactions must correlate to the difference in their separate states. They are known as photons, and are the particles we now know.
Hydrogen
The spectrum of hydrogen covers most of the electromagnetic spectrum. When electrons absorb radiation, they then have more energy. Electrons do not keep this energy though. They will lose it. The question is how fast.
What is the purpose of astronomical spectroscopy?
Background. Astronomical spectroscopy is used to measure three major bands of radiation: visible spectrum, radio, and X-ray. While all spectroscopy looks at specific areas of the spectrum, different methods are required to acquire the signal depending on the frequency.
What wavelengths do X-rays and ultraviolet spectroscopy use?
Ozone (O 3) and molecular oxygen (O 2) absorb light with wavelengths under 300 nm, meaning that X-ray and ultraviolet spectroscopy require the use of a satellite telescope or rocket mounted detectors. Radio signals have much longer wavelengths than optical signals, and require the use of antennas or radio dishes.
What are cometary spectra?
The spectra of comets consist of a reflected solar spectrum from the dusty clouds surrounding the comet, as well as emission lines from gaseous atoms and molecules excited to fluorescence by sunlight and/or chemical reactions. For example, the chemical composition of Comet ISON was determined by spectroscopy due to the prominent emission lines of cyanogen (CN), as well as two- and three-carbon atoms (C 2 and C 3 ). Nearby comets can even be seen in X-ray as solar wind ions flying to the coma are neutralized. The cometary X-ray spectra therefore reflect the state of the solar wind rather than that of the comet.
How did Isaac Newton observe the solar spectrum?
Physicists have been looking at the solar spectrum since Isaac Newton first used a simple prism to observe the refractive properties of light. In the early 1800s Joseph von Fraunhofer used his skills as a glassmaker to create very pure prisms, which allowed him to observe 574 dark lines in a seemingly continuous spectrum. Soon after this, he combined telescope and prism to observe the spectrum of Venus, the Moon, Mars, and various stars such as Betelgeuse; his company continued to manufacture and sell high-quality refracting telescopes based on his original designs until its closure in 1884. : 28–29
What are the absorption bands of a planet?
The reflected light of a planet contains absorption bands due to minerals in the rocks present for rocky bodies, or due to the elements and molecules present in the atmosphere. To date over 3,500 exoplanets have been discovered. These include so-called Hot Jupiters, as well as Earth-like planets. Using spectroscopy, compounds such as alkali metals, water vapor, carbon monoxide, carbon dioxide, and methane have all been discovered.
What are the spectral features of interstellar medium?
Their spectral features are generated by transitions of component electrons between different energy levels, or by rotational or vibrational spectra. Detection usually occurs in radio, microwave, or infrared portions of the spectrum. The chemical reactions that form these molecules can happen in cold, diffuse clouds or in dense regions illuminated with ultraviolet light. Polycyclic aromatic hydrocarbons such as acetylene (C 2 H 2) generally group together to form graphites or other sooty material, but other organic molecules such as acetone ( (CH 3) 2 CO) and buckminsterfullerenes (C 60 and C 70) have been discovered.
What is radio astronomy?
He built a radio antenna to look at potential sources of interference for transatlantic radio transmissions. One of the sources of noise discovered came not from Earth, but from the center of the Milky Way, in the constellation Sagittarius. In 1942, JS Hey captured the sun's radio frequency using military radar receivers. Radio spectroscopy started with the discovery of the 21-centimeter H I line in 1951.
Why is infrared spectroscopy conducted in space?
Infrared spectroscopy is conducted in space because the Earth’s atmosphere blocks out most infrared wavelengths in addition to producing its own , which can overwhelm celestial sources. The infrared part of the electromagnetic spectrum – which lies between 0.75 and 300 μm - is where the emission and absorption lines of virtually all molecules, ...
What is Infrared Spectroscopy?
Ground-based infrared spectroscopy has a much longer history than space-based infrared spectroscopy, and as a result, many of the terms used relate to the windows in the Earth’s atmosphere where lower absorption spectroscopy makes astronomy feasible. Infrared spectroscopy is conducted in space because the Earth’s atmosphere blocks out most infrared wavelengths in addition to producing its own, which can overwhelm celestial sources.
How much of the universe's light is absorbed by stars?
Around half of all starlight produced and emitted throughout the Universe’s 13.8-billion-year history has been absorbed and re-emitted as infrared light; most atoms and molecules - except hydrogen and helium – have their origins in the stars.
What is the Spitzer telescope?
The NASA Spitzer Space Telescope, launched in 2003, features the Infrared Array Camera, which operated in the mid- and near-infrared and used over 65,000 pixels across each of its four detectors until it ran out of coolant in 2017. It also features the Infrared Spectrograph, which provides high- and low-resolution spectroscopy at mid-infrared wavelengths; it has, for example, collected data that displays a strong signature of water vapor in the disk of gas and dust surrounding a young star.
When was infrared spectroscopy first used?
Infrared spectroscopy originated in the 1830s after German-born British astronomer William Herschel discovered the existence of infrared radiation while studying sunlight. It wasn’t until the 1920s that the first systematic infrared observations of stellar objects other than the sun and moon were made by the American astronomers W.W. Coblentz, Edison Pettit, and Seth B. Nicholson.
How much light can we see in the near infrared?
The near-infrared: approximately 0.75 – 5 μm wavelength. This light behaves similarly to visible wavelengths due to its proximity (our eyes can see up to 0.75 μm).
What happens to stars during their lifetime?
Throughout their lifetime, stars produce heavier elements, which are expelled into interstellar space as the star explodes during its final phase of life. Some of these atoms will join to form molecules; together these form the basic building blocks of all matter. Deducing what atoms and molecules are present, their distribution, abundance and environment is necessary to understanding the Universe.
How does spectroscopy work in astronomy?
Astronomical spectra can be analyzed to determine the chemical composition of stars, galaxies, and nebulae, as well as the distance to stars, their temperatures, and sizes. Of course, the excitement of revealing new potentially habitable planets, such as the recent discovery by NASA of seven Earth-sized planets around a single star is also brought to you by spectroscopy!
Why is spectroscopy important in art?
Beyond fundamental research, there’s a role for spectroscopy in the world of art. Spectroscopy allows art conservators to non-invasively identify the materials used in works of art. The unique chemical signatures detected by Raman and infrared spectroscopy show conservators whether and how their artwork is degrading, thus providing the information needed to preserve old art for future generations to enjoy.
What was the first tool to be used to measure spectra?
But it wasn’t until the 20 th century, when quantum mechanics revolutionized the field of spectroscopy, that a myriad of spectroscopic techniques emerged for researchers. One of the most significant tools to be developed for the field of spectroscopy in the mid-20 th century is the laser .
What is the scientific term for measuring the spectrum of light?
A spectrum is measured by the scientific technique known as spectroscopy, and unless you’re already familiar with the term, this may compel you to ask: what is spectroscopy? The short answer is that spectroscopy refers to the study of the interaction between light and matter. Today, the field of spectroscopy is incredibly broad and advanced, with applications in not just astronomy but also chemistry, physics, biology, environmental science, and even art!
When was spectroscopy invented?
The history of spectroscopy goes back to the 17 th century , when Isaac Newton showed that a prism could separate white light into several components that we perceive as colors. The different colors correspond to different wavelengths (and thus energies) of light. In very general terms, a spectrum shows the intensity of each of these wavelengths.
Who said spectroscopy can answer the question "are we alone"?
Astrophysicist Garik Israelian started a TED talk in 2009 with the following claim: “Spectroscopy can probably answer the question: ‘is there anybody out there? Are we alone?’” So in addition to the aforementioned applications, how might spectroscopy answer this compelling question? You can read or listen to the full TED talk here, but I’ll leave you with my own paraphrasing of his statement. Israelian says that the answer to the question “Are we alone?” will come from a spectrum that shows the same molecules in the atmosphere that are essential for life on Earth.
How does spectroscopy help scientists?
Spectroscopy can be very useful in helping scientists understand how an object like a black hole, neutron star, or active galaxy produces light, how fast it is moving, and what elements it is composed of. Spectra can be produced for any energy of light, from low-energy radio waves to very high-energy gamma rays.
What Can Scientists Learn From a Spectrum?
Three types of spectra: continuous, emission line and absorption. (Credit: NASA's Imagine the Universe)
What is the study of a star called?
This type of study is called spectroscopy . The science of spectroscopy is quite sophisticated. From spectral lines astronomers can determine not only the element, but the temperature and density of that element in the star. The spectral line also can tell us about any magnetic field of the star.
What is the electromagnetic spectrum?
It covers all energies of light, extending from low-energy radio waves, to microwaves, to infrared, to optical light, to ultraviolet, to very high-energy X-rays and gamma rays. The full electromagnetic spectrum. (Credit: NASA's Imagine the Universe) Tell Me More About the Electromagnetic Spectrum!
What is a spectrum?
A spectrum is simply a chart or a graph that shows the intensity of light being emitted over a range of energies. Have you ever seen a spectrum before? Probably. Nature makes beautiful ones we call rainbows. Sunlight sent through raindrops is spread out to display its various colors (the different colors are just the way our eyes perceive radiation with slightly different energies).
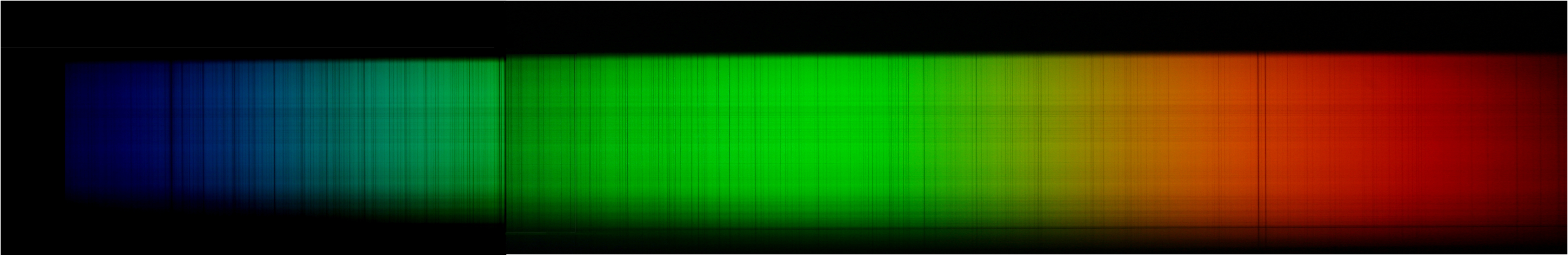
Properties of Light
The Value of Stellar Spectra
- When Newton described the laws of refraction and dispersion in optics, and observed the solar spectrum, all he could see was a continuous band of colors. If the spectrum of the white light from the Sun and stars were simply a continuous rainbow of colors, astronomers would have little interest in the detailed study of a star’s spectrum once they had learned its average surface tem…
Types of Spectra
- In these experiments, then, there were three different types of spectra. A continuous spectrum (formed when a solid or very dense gas gives off radiation) is an array of all wavelengths or colors of the rainbow. A continuous spectrum can serve as a backdrop from which the atoms of much less dense gas can absorb light. A dark line, or absorption spe...
Glossary
- absorption spectrum: a series or pattern of dark lines superimposed on a continuous spectrum continuous spectrum: a spectrum of light composed of radiation of a continuous range of wavelengths or colors, rather than only certain discrete wavelengths dispersion: separation of different wavelengths of white light through refraction of different amounts emission spectrum: …
Overview
Astronomical spectroscopy is the study of astronomy using the techniques of spectroscopy to measure the spectrum of electromagnetic radiation, including visible light, ultraviolet, X-ray, infrared and radio waves that radiate from stars and other celestial objects. A stellar spectrum can reveal many properties of stars, such as their chemical composition, temperature, density, mass, distance and …
Background
Astronomical spectroscopy is used to measure three major bands of radiation in the electromagnetic spectrum: visible light, radio waves, and X-rays. While all spectroscopy looks at specific bands of the spectrum, different methods are required to acquire the signal depending on the frequency. Ozone (O3) and molecular oxygen (O2) absorb light with wavelengths under 300 nm, meaning t…
Stars and their properties
Newton used a prism to split white light into a spectrum of color, and Fraunhofer's high-quality prisms allowed scientists to see dark lines of an unknown origin. In the 1850s, Gustav Kirchhoff and Robert Bunsen described the phenomena behind these dark lines. Hot solid objects produce light with a continuous spectrum, hot gases emit light at specific wavelengths, and hot sol…
Galaxies
The spectra of galaxies look similar to stellar spectra, as they consist of the combined light of billions of stars.
Doppler shift studies of galaxy clusters by Fritz Zwicky in 1937 found that the galaxies in a cluster were moving much faster than seemed to be possible from the mass of the cluster inferred from the visible light. Zwicky hypothesized that there must be a great deal of non-luminous matter in t…
Interstellar medium
The interstellar medium is matter that occupies the space between star systems in a galaxy. 99% of this matter is gaseous - hydrogen, helium, and smaller quantities of other ionized elements such as oxygen. The other 1% is dust particles, thought to be mainly graphite, silicates, and ices. Clouds of the dust and gas are referred to as nebulae.
There are three main types of nebula: absorption, reflection, and emission nebulae. Absorption (or …
Motion in the universe
Stars and interstellar gas are bound by gravity to form galaxies, and groups of galaxies can be bound by gravity in galaxy clusters. With the exception of stars in the Milky Way and the galaxies in the Local Group, almost all galaxies are moving away from us due to the expansion of the universe.
The motion of stellar objects can be determined by looking at their spectrum. …
Planets, asteroids, and comets
Planets, asteroids, and comets all reflect light from their parent stars and emit their own light. For cooler objects, including solar-system planets and asteroids, most of the emission is at infrared wavelengths we cannot see, but that are routinely measured with spectrometers. For objects surrounded by gas, such as comets and planets with atmospheres, further emission and absorption happens at specific wavelengths in the gas, imprinting the spectrum of the gas on th…
See also
• Atomic and molecular astrophysics
• Emission spectrum
• Gunn-Peterson trough
• Lyman-alpha forest