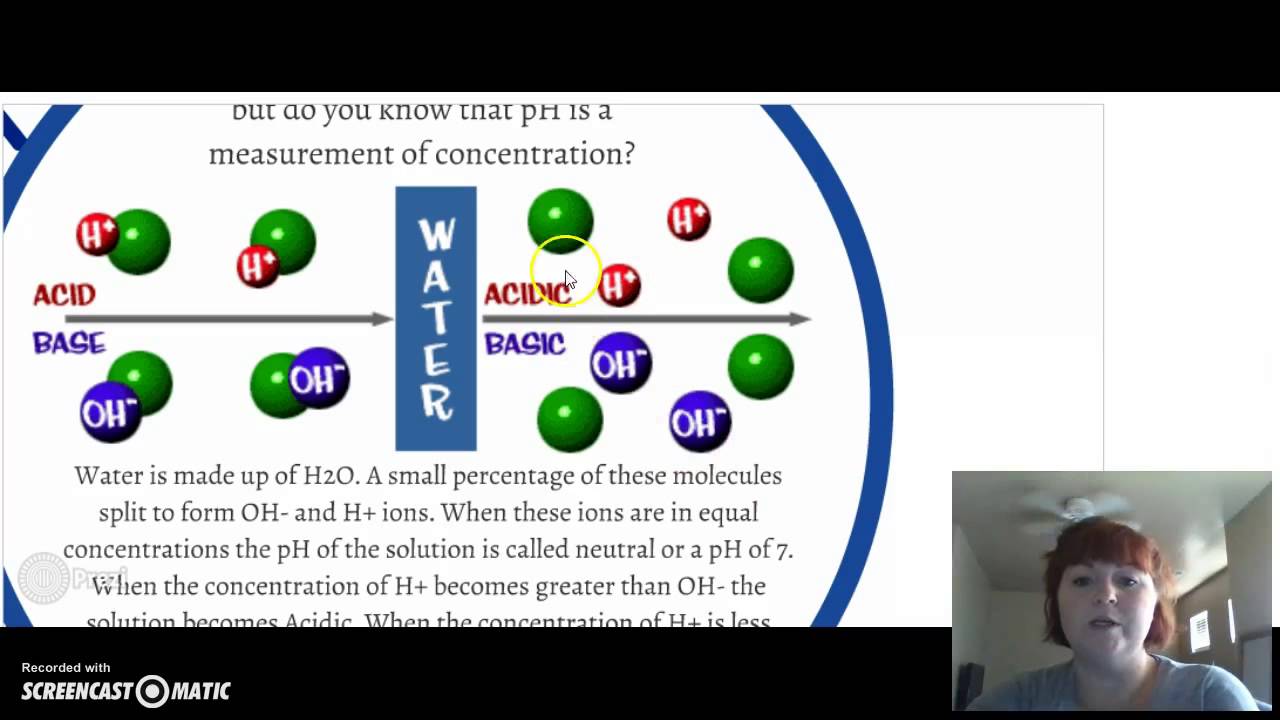
What kind of ions do acids release?
The Arrhenius theory defines an acid as substance that dissociates to produce H+ ions in solution and a base as a substance that dissociates to produce OH- ions in solution, as per the answer to your question. 2. The Bronsted-Lowery theory defines an acid as a proton donor and a base as a proton acceptor.
What do ions do acids release?
What ion do Acids release in solution hydrogen? An acid produces hydrogen ions in solution because it reacts with the water molecules by giving a proton to them. When hydrogen chloride gas dissolves in water to produce hydrochloric acid, the hydrogen chloride molecule gives a proton (a hydrogen ion) to a water molecule. Click to see full answer.
Do acids lose or gain hydrogen ions?
Type in your name, wait 107 seconds, brace yourself. An acid is anything that loses a hydrogen ion (H+). Acids, on the other hand, cannot lose hydrogen ions to empty space; they can only lose them to other substances having strong negative regions that attract hydrogen ions.
What ion does an acid release?
Most acids contain a hydrogen atom bonded that can release (dissociate) to yield a cation and an anion in water. The higher the concentration of hydrogen ions produced by an acid, the higher its acidity and the lower the pH of the solution.

What ion do acids release in solutions?
hydrogen ions (H+)Acids are chemical compounds that release hydrogen ions (H+) when placed in water. For example, when hydrogen chloride is placed in water, it releases its hydrogen ions and the solution becomes hydrochloric acid. Bases are chemical compounds that attract hydrogen atoms when they are placed in water.
Do acids release OH ions in a solution?
Acids are substances that when dissolved in water release hydrogen ions, H+(aq). Bases are substances that react with and neutralise acids, producing water. When dissolved, bases release hydroxide ions, OH-(aq) into solution. Water is the product of an acid and base reacting.
What ion do bases release in solution?
A base is a substance that releases hydroxide ions (OH-) into solution. They have a high pH - above 7. Soluble bases are called ALKALI. pH is a measure of the hydrogen ion concentration in a solution.
Do bases release hydrogen ions into solution?
Bases do not release H+ but accept them. There are three definitions for a base: Bases are those substances that can donate OH- Bases are those substances that can accept H+
Is acid H+ or OH?
An acid is a substance that releases H+ when dissolved in water. Basic solutions have a low H+ concentration. A base is a substance that releases OH- when dissolved in water.
Do acids have OH or H?
Acid: A solution that has an excess of H+ ions. It comes from the Latin word acidus, which means "sharp" or "sour". Base: A solution that has an excess of OH- ions.
Why do acids release H+ ions?
An acid produces hydrogen ions in solution because it reacts with the water molecules by giving a proton to them.
What ion do acids release in solution quizlet?
Acids produce hydrogen ions (H+) and bases produce hydroxide ions (OH-) when dissolved in solution.
What do acids do in solution?
For our purposes, an acid is a substance that increases the concentration of hydrogen ions (H +start superscript, plus, end superscript) in a solution, usually by donating one of its hydrogen atoms through dissociation.
Do acids lose or gain hydrogen ions?
To determine whether a substance is an acid or a base, count the hydrogens on each substance before and after the reaction. If the number of hydrogens has decreased that substance is the acid (donates hydrogen ions). If the number of hydrogens has increased that substance is the base (accepts hydrogen ions).
How are H+ ions formed?
A hydrogen ion is created when a hydrogen atom loses or gains an electron. A positively charged hydrogen ion (or proton) can readily combine with other particles and therefore is only seen isolated when it is in a gaseous state or a nearly particle-free space.
Do all acids produce hydrogen ions in water?
Acids dissociate in water or aqueous solutions to form ions. These are responsible for the conduction of electricity. Acids don't dissociate hydrogen ions in absence of water. Therefore, we can say that acids produce ions only in aqueous solutions.
What happens to the OH ions in a solution?
The hydroxyl ions (OH-) released will combine with any hydrogen ions (H+) in the solution to form water molecules (OH- + H+ = H2O), so we can also define a base as a substance that takes or accepts hydrogen ions (H+) already present in the solution.
Do acids lose or gain hydrogen ions?
To determine whether a substance is an acid or a base, count the hydrogens on each substance before and after the reaction. If the number of hydrogens has decreased that substance is the acid (donates hydrogen ions). If the number of hydrogens has increased that substance is the base (accepts hydrogen ions).
What ion do acids release in solution quizlet?
Acids produce hydrogen ions (H+) and bases produce hydroxide ions (OH-) when dissolved in solution.
Do bases release hydroxide ions in water?
Bases can be defined as substances which when dissoved in water produce hydroxide ions(OH-).
What ions do acids form in water?
When dissolved in water, acids donate hydrogen ions (H+). Hydrogen ions are hydrogen atoms that have lost an electron and now have just a proton, giving them a positive electrical charge. Bases, on the other hand, mixed with water yield hydroxide ions (OH-).
What is ionic product of water?
The ion-product of water is the mathematical product of the concentration of hydrogen ions and hydroxide ions. Note that H 2 O is not included in the ion-product expression because it is a pure liquid. The value of is very small, in accordance with a reaction that favors the reactants.
Which species release hydrogen ions into a solution?from brainly.com
Whereas bases are the species that release hydroxide or ions into a solution. Thus, we can conclude that acids release hydrogen ions in solution.
What is the pH of a solution?from quizlet.com
A solution has a pH of 8. Which best describes the solution?
Which acid neutralizes the acid entering the esophagus?from quizlet.com
The antacid is basic and will neutralize the acid entering the esophagus.
What binds with OH to make water?from quizlet.com
H+ binds with OH- to make water.