
Why does the body produce lactic acid?
Key points
- Lactic acid is the waste product produced during anaerobic respiration.
- Running fast can lead to a build-up of lactic acid in your muscles, causing cramp.
- Lactic acid also causes tooth decay.
What can lactic acid do to the body?
Truth be told, if you are well trained, lactic acid can enhance your performance, as your body gets to be efficient at burning it with oxygen for extra energy. Apparently, the increase in lactic acid is higher when you are not fit.
Is lactic acid really that bad?
That god-awful burning you feel is the buildup of hydrogen in the form of lactic acid. Pyruvate becomes lactate, Hamilton explains, when there isn’t enough oxygen to create more ATP. But don’t stress: that lactate can eventually be metabolized for energy. This means, despite what most people believe, lactate isn’t really a bad thing.
What is the natural source of lactic acid?
Lactic acid, or lactate, is a chemical byproduct of anaerobic respiration — the process by which cells produce energy without oxygen around. Bacteria produce it in yogurt and our guts. Lactic acid is also in our blood, where it's deposited by muscle and red blood cells.
See more

What converts milk into lactic acid?
Lactobacillus Bacteria in Milk that changes Milk to Curd. When milk is heated to a temperature of 30-40 degrees centigrade and a small amount of old curd added to it, the lactobacillus in that curd sample gets activated and multiplies. These convert the lactose into lactic acid. Q.
How does lactose turn into lactic acid?
Fermentation is the process by which the Lactobacillus bacteria breakdown the milk sugar, lactose into lactic acid. Due to the production of acid, its pH decreases. This breaks down the milk protein, separating it from other constituents. This process is called coagulation.
Is lactic acid found in milk?
The real acidity of milk is due to lactic acid.
What is the process of producing lactic acid?
Lactic acid is an organic compound produced via fermentation by different microorganisms that are able to use different carbohydrate sources. Lactic acid bacteria are the main bacteria used to produce lactic acid and among these, Lactobacillus spp. have been showing interesting fermentation capacities.
What is the purpose of lactic acid in milk?
Lactic acid bacteria (LAB) are one of the most significant groups of probiotic organisms, commonly used in fermented dairy products. Among other benefits, these microorganisms can enhance lactose digestion, stimulate the immune system, and prevent and treat diarrhea [5].
What is the difference between lactate and lactic acid?
To understand the difference you have to look at the chemistry. An acid is a substance that is able to donate hydrogen ions (H+). Lactic acid is such a substance. When it donates a hydrogen ion the resultant product is referred to as the conjugate base of the acid and in this case is Lactate.
Does boiled milk has lactic acid?
Milk heated at 120 ° C. for 30 minutes contained 202.3 mg. lactic acid and 42.1 mg. formic acid per 100 ml.
How much lactic acid is in milk?
It has been stated many times in the literature that fresh milk contains no lactic acid. Our results indicate that if fresh milk contains lactic acid, the amount present is not over about 0.002 per cent.
Which type of acid are found in milk?
lactic acidThe weak acid present in milk is lactic acid.
What is the pH of lactic acid?
ACIDS AND BASES The pH of the lactic acid solution is 2.43.
What is lactic acid made of?
The natural production method features bacterial fermentation of carbohydrates (sugars, starches). Lactic acid is made from beet sugar, cane sugar, corn and tapioca. Lactic acid is frequently used as an exfoliant and in anti-wrinkle products, and in our body wash, it functions as a natural preservative.
How can you make lactic acid at home?
HOW TO MAKE LAB FROM MILK ? Put rice-washed water 15 to 20 cm deep in a jar. Cover the mouth of the jar with handmade paper and leave in shade. Lactic acid bacteria will propagate at 23 to 25 C, and the solution will start to smell sour.
Does lactose break down to lactic acid?
Lactic Acid Formation. For many cheeses, lactose ends up as lactic acid. This is accomplished by the metabolic processes of the bacterial starter culture the cheesemaker uses.
Is lactic acid is formed from lactose?
Many people assume that lactic acid comes from animal products because the first word in the term sounds similar to lactose, a sugar naturally found in cow's milk and dairy products. Adding to the confusion, the prefix “lac-” is Latin for “milk.” However, lactic acid is not milk, nor does it contain milk.
Do lactose intolerant people produce lactic acid?
Lactose intolerance is an impairment of your body's ability to produce lactase, an enzyme that digests lactose, a sugar found in milk. If your body can't digest that sugar, the lactose may ferment in your intestines and produce lactic acid.
Can lactose intolerant have lactic acid?
Some ingredients may sound like they contain lactose when they do not, such as lactic acid, sodium lactate and cocoa butter. These ingredients do not need to be avoided if you're lactose intolerant.
1. Which Food Contains Lactic Acid?
Lactic acid is present in a large amount of commonly consumed foods. It occurs in the food as a result of fermentation or in the form of an additiv...
2. Why Does Lactic Acid Build-Up?
Lactic acid builds up in your body when there is inadequate oxygen in the muscles to produce energy. Typically, the muscles tend to break down gluc...
3. What Does Lactic Acid Do to Your Body?
When your body doesn’t receive enough oxygen to produce energy, then it makes lactic acid. It can cause a burning feeling in the muscles you are us...
4. What are Some Properties of Acids and Bases?
An acid is a chemical species capable of donating hydrogen ions to another substance. A base is a molecule that accepts electrons or releases hydro...
5. How to Study the Acids and Bases Chapter?
The chapter Acids and Bases is an important chapter of chemistry for students as it sets a base for other chapters in the subject and higher classe...
1. What is Lactic Acid Formula
Ans: Lactic acid is an organic acid with having the chemical formula C3H6O3. Its IUPAC name is 2-Hydroxypropanoic acid.
2. What is lactic acid made from?
Ans: Lactic acid is made from the fermentation of molasses and other carbohydrates like corn, beet sugar, cane sugar, and tapioca.
3. What reacts with lactic acid?
Ans: After doing lots of physical activities, we often feel tired, fatigue, or even muscle cramps in our bodies. The reason behind this is the accu...
4. What is the colour of lactic acid?
Ans: Lactic acid is white in solid-state and colourless in the liquid solution.
5. Is lactic acid a solid, liquid, or gas at room temperature?
Ans: Lactic acid exists as a water-soluble liquid at room temperature but, it gets freeze into a white solid at a lower temperature, i.e., around 1...
What is lactic acid?
Lactic acid is used as a food preservative, curing agent, and flavoring agent. It is an ingredient in processed foods and is used as a decontaminant during meat processing. Lactic acid is produced commercially by fermentation of carbohydrates such as glucose, sucrose, or lactose, or by chemical synthesis.
Where is lactic acid found?
Lactic acid is found primarily in sour milk products, such as kumis, laban, yogurt, kefir, and some cottage cheeses. The casein in fermented milk is coagulated (curdled) by lactic acid. Lactic acid is also responsible for the sour flavor of sourdough bread.
What is the process of fermentation in wine?
In winemaking, a bacterial process, natural or controlled, is often used to convert the naturally present malic acid to lactic acid, to reduce the sharpness and for other flavor-related reasons. This malolactic fermentation is undertaken by lactic acid bacteria .
What is the sourdough bread that has lactic acid?
Lactic acid is also responsible for the sour flavor of sourdough bread. In lists of nutritional information lactic acid might be included under the term "carbohydrate" (or "carbohydrate by difference") because this often includes everything other than water, protein, fat, ash, and ethanol.
What is the process of lactic acid fermentation?
In industry, lactic acid fermentation is performed by lactic acid bacteria, which convert simple carbohydrates such as glucose, sucrose, or galactose to lactic acid. These bacteria can also grow in the mouth; the acid they produce is responsible for the tooth decay known as caries.
What is a mixture of lactic acid and ethanol called?
A mixture of the two in equal amounts is called DL -lactic acid, or racemic lactic acid. Lactic acid is hygroscopic. DL -Lactic acid is miscible with water and with ethanol above its melting point, which is around 16, 17 or 18 °C. D -Lactic acid and L -lactic acid have a higher melting point.
How is racemic lactic acid made?
Racemic lactic acid is synthesized industrially by reacting acetaldehyde with hydrogen cyanide and hydrolysing the resultant lactonitrile. When hydrolysis is performed by hydrochloric acid, ammonium chloride forms as a by-product; the Japanese company Musashino is one of the last big manufacturers of lactic acid by this route. Synthesis of both racemic and enantiopure lactic acids is also possible from other starting materials ( vinyl acetate, glycerol, etc.) by application of catalytic procedures.
How is lactic acid produced?
It gets produced by the muscles during intense activity. Lactic acid is soluble in water. It looks white in its solid-state and becomes colourless in the liquid state. Milk acid is another name of lactic acid. When lactose or milk sugar undergoes fermentation, the lactic acid gets produced.
Where does lactic acid come from?
Lactic acid gets produced in yoghurt by some bacteria. It is also present in your gut and blood. Your muscles and red blood cells often deposit the lactate into your blood. So, lactic acid is an organic one. It’s a chiral molecule, and it has two optical isomers, which are L-lactic acid and D-lactic acid.
What is Lactic Acid?
Lactic acid is one of the organic acids. The chemical formula of the lactic acid is C3H6O3. It has two optical isomers, Levo and Dextro, making itself a chiral molecule. L-isomers are commonly present among living organisms. The lactic acid has a significant part in various biochemical processes. It gets produced by the muscles during intense activity.
What is the process of producing lactic acid?
Lactic acid, also known as lactate, is a chemical byproduct of anaerobic respiration. It refers to a process where cells produce energy without having oxygen around. Lactic acid gets produced in yoghurt by some bacteria. It is also present in your gut and blood. Your muscles and red blood cells often deposit the lactate into your blood. So, lactic acid is an organic one. It’s a chiral molecule, and it has two optical isomers, which are L-lactic acid and D-lactic acid. The presence of carboxyl group adjacent to the hydroxyl group makes lactic acid an alpha-hydroxy acid. In this article, you can learn about lactic acid structure, its definition, uses, and sources.
Why does lactic acid build up in the body?
Lactic acid builds up in your body when there is inadequate oxygen in the muscles to produce energy. Typically, the muscles tend to break down glucose and glycogen to produce energy. It is an anaerobic metabolism. When your body moves, the body uses oxygen to get energy.
What is the molar mass of lactic acid?
In solid form, you can find it in the white powder. The molecular weight or molar mass of lactic acid is 90.08 g/mol. And it’s PH level is 3.51 per 1 mM of lactic acid.
Where can I find lactic acid?
You can find lactic acid in pickled vegetables, beer, wine, sourdough bread, kimchi, and fermented soy products like soy sauce.
How is lactic acid made?
From Acetaldehyde Lactic acid is prepared from acetaldehyde by following simple two-step synthesis; firstly, it involves nucleophilic addition of H C N to the carbonyl group of acetalde hyde and secondly, aqueous hydrolysis of the nitrile takes place to yield the carboxylic acid product, i.e., lactic acid.
Where is lactic acid produced?
In the human body, lactic acid is primarily produced in muscle cells and red blood cells during intense exercise. When the rate of demand for energy in the body is high:
What is Lactic Acid?
Lactic acid is an organic acid. It is the main constituent of milk. Hence, also known as milk acid. It contains “carboxylic acid” as a functional group. Lactic acid is a chemical byproduct of anaerobic respiration (which cells produce energy without oxygen).
Why is lactic acid important in dairy products?
3. Lactic acid plays an essential role in making dairy products such as yogurt, cheese, etc., as it helps in the coagulation of milk protein. The presence of lactic acid or lactate in milk is due to the fermentation of lactose caused mainly by lactic acid bacteria.
What is the reaction between lactic acid and sodium bicarbonate?
Lactic acid reacts with Sodium bicarbonate ( N a H C O 3) to give salt (sodium lactate), water, and release carbon dioxide gas. This reaction can be used to test the presence of the carboxylic acid group in lactic acid.
What is the reaction between ethanol and lactic acid?
Lactic acid reacts with ethanol ( C 2 H 5 O H) In the presence of sulphuric acid, to form ethyl lactate and a water molecule. It resembles an esterification reaction where acid and alcohol react to form an ester.
Why does lactic acid have hydrogen bonds?
Lactic acid has intramolecular hydrogen bonding because of adjacent carboxyl and hydroxyl groups. It deprotonates in its aqueous solution to form its conjugate base, lactate.
Why does lactic acid increase?
Lactic acid levels increase in some of the following situations, according to University of Michigan Medicine: During strenuous exercise (this is the most common reason among healthy adults). When someone experiences heart failure, liver failure or pulmonary embolism.
Why is lactate a natural acid?
That’s because lactate can be converted to energy without using oxygen.
Why is lactic acid produced during exercise?
Lactic acid is produced in higher-than-normal amounts during tough aerobic exercise, since intense physical activity causes the muscles to need more oxygen. When exercise is vigorous enough to cause a high demand for oxygen that the lungs and heart can not keep up with, then lactic acid builds up in the blood.
What is it called when lactic acid levels rise?
However, when lactic acid levels rise significantly, this is called lactic acidosis, which is considered to be life-threatening.
How to prevent lactic acidosis?
What can you do to perform more like a highly trained athlete? As explained more below, some of the ways you can prevent too much lactic acid — along with lactic acidosis — include gradually building up exercise intensity, staying hydrated, stretching, taking enough rest days, and fueling with good nutrition before and after workouts.
How long does it take for lactic acid to peak?
Soreness, stiffness and loss of strength and range of motion usually peak about one to three days after the extreme exercise takes place, but how intense these symptoms are does not depend on how much lactic acid accumulates during exercise.
What is the role of lactic acid in the body?
Role in Body and Exercise. The definition of lactic acid is “an organic acid (C 3 H 6 O 3) present especially in muscle tissue as a by-product of anaerobic glycolysis, produced in carbohydrate matter usually by bacterial fermentation, and used especially in food and medicine and in industry.”. In other words, it’s a natural acid produced in ...
When does lactic acid form?
It is formed when the body has no oxygen. So when the body does have oxygen in the resting phase the lactic acid can be converted back into energy though the aerobic energy system and kreb cycle.
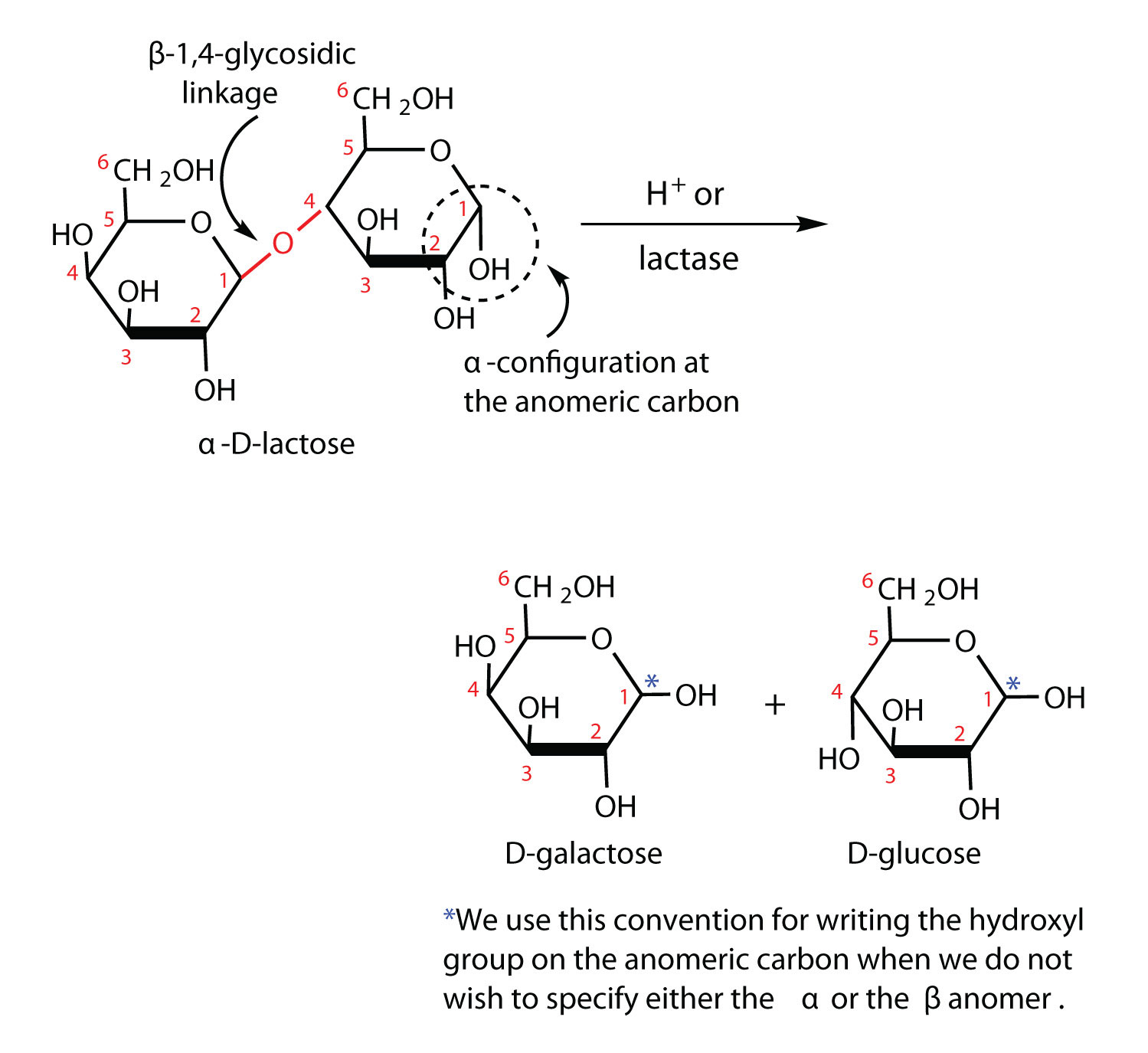
What bacteria take lactose from milk and turn it into lactic acid?
You partly answered your question. Lactic acid bacteria (Lactobacillus) take lactose from milk and turn it into lactic acid. The resulting drop in pH causes proteins in the milk to coagulate, forming a curd. That is th first step in the manufacture of cheese and other fermented milk products.
What is the fermentation of grapes into wine?
fermentation of grapes into wine involves yeast and lactic acid bacteria. Malolactic fermentation is one means of adding complexity to a wine. Following the primary fermentation of the sugar in the grapes to alcohol, bacteria convert the grape acids into malic acid and lactic acid. Lactic acid is less sour than malic acid. It adds to the complexity and flavor to a good wine.
How to make lactose sugar into lactic acid?
Simple — introduce lactic acid bacteria (LAB) into your room temperature milk and they will convert the lactose sugar into lactic acid.
Why is lactate in milk?
The presence of lactic acid or lactate in milk is due to the fermentation of lactose caused mainly by lactic bacteria. Generally speaking, just-milked milk does not contain lactic acid, but this increases after a while and its concentration is closely correlated to the total bacterial charge. Once formed, however, it is present as lactate. The pH of milk is about 6.6. The pKa of lactic acid is about 3.86. The equilibrium for lactic acid/lactate is
What is the ratio of lactate to lactic acid?
So the ratio of lactate to lactic acid will be 550:1, so mostly lactate.
What is lactic acid?
Lactic acid are the result of decomposition of gylcogen stored in our body.
What are the constituents of milk?
Print this chapter. The principal constituents of milk are water, fat, proteins, lactose (milk sugar) and minerals (salts). Milk also contains trace amounts of other substances such as pigments, enzymes, vitamins, phospholipids (substances with fatlike properties), and gases. The residue left when water and gases are removed is called ...
What are the components of milk fat?
Milk fat consists of triglycerides (the dominating components), di- and monoglycerides, fatty acids, sterols, carotenoids (giving the yellow colour of the fat) and vitamins (A, D, E, and K). Trace elements are minor components. The composition of a milk fat globule is outlined in Figure 2.16.
How are proteins classified in milk?
The proteins can be classified in various ways according to their chemical or physical properties and their biological functions. The old way of grouping milk proteins into casein, albumin and globulin has given way to a more adequate classification system. Table 2.5 shows an abridged list of milk proteins according to a modern system. Minor protein groups have been excluded for the sake of simplicity.#N#Whey protein is a term often used as a synonym for milk-serum proteins, but it should be reserved for the proteins in whey from the cheese making process. In addition to milk-serum proteins, whey protein also contains fragments of casein molecules. Some of the milk-serum proteins are also present in whey in lower concentrations than in the original milk. This is due to heat denaturation during pasteurization of the milk prior to cheese-making. The three main groups of proteins in milk are distinguished by their widely different behaviour and form of existence. The caseins are easily precipitated from milk in a variety of ways, while the serum proteins usually remain in solution. The fat-globule membrane proteins adhere, as the name implies, to the surface of the fat globules and are only released by mechanical action, e.g. by churning cream into butter.
What are some examples of fat in water?
Milk and cream are examples of fat-in-water (or oil-in-water) emulsions. The milk fat exists as small globules or droplets dispersed in the milk serum, Figure 2.15. Their diameters range from 0.1 to 20 µm (1 µm = 0.001 mm). The average size is 3 – 4 µm and there are some 10 10 globules per ml.#N#The emulsion is stabilized by a very thin membrane only 10-20 nm thick (1 nm = 10 –9 m) which surrounds the globules and has a complicated composition.#N#Milk fat consists of triglycerides (the dominating components), di- and monoglycerides, fatty acids, sterols, carotenoids (giving the yellow colour of the fat) and vitamins (A, D, E, and K). Trace elements are minor components. The composition of a milk fat globule is outlined in Figure 2.16.#N#The membrane consists of phospholipids, lipoproteins, cerebrosides, proteins, nucleic acids, enzymes, trace elements (metals) and bound water. It should be noted that the composition and thickness of the membrane are not constant, because components are constantly being exchanged with the surrounding milk serum.#N#As the fat globules are not only the largest particles in the milk but also the lightest (density at 15.5 °C = 0.93 g/cm 3 ), they tend to rise to the surface when milk is left to stand in a vessel for a while, Figure 2.17.#N#The rate of rise follows Stokes’ Law, but the small size of the fat globules makes creaming a slow process. Cream separation can, however, be accelerated by aggregation of fat globules under the influence of a protein called agglutinin. These aggregates rise much faster than individual fat globules. The aggregates are easily broken up by heating or mechanical treatment. Agglutinin is denatured at time-temperature combinations such as 75 °C/ 2 min and the possibility of aggregation disappears.
How to detect phosphatase in milk?
The presence of phosphatase in milk can be detected by adding a phosphoric-acid ester and a reagent that changes colour when it reacts with the liberated alcohol. A change in colour reveals that the milk contains phosphatase.#N#Phosphatase is destroyed by ordinary pasteurization (72 °C for 15 – 20 seconds), so the phosphatase test can be used to determine whether the pasteurization temperature has actually been attained. The routine test used in dairies is called the phosphatase test according to Scharer.#N#The phosphatase test should preferably be performed immediately after heat treatment. In other cases, the milk must be chilled to below + 5 °C and kept at that temperature until analysed. The analysis should be carried out the same day, otherwise a phenomenon known as reactivation may occur, i.e. an inactivated enzyme becomes active again and gives a positive test reading. Cream is particularly susceptible in this respect.
How does catalase work?
Catalase splits hydrogen peroxide into water and free oxygen. By determining the amount of oxygen that the enzyme can release in milk, it is possible to estimate the catalase content of the milk and learn whether or not the milk has come from an animal with a healthy udder. Milk from diseased udders has a high catalase content, while fresh milk from a healthy udder contains only an insignificant amount. There are, however, many bacteria that produce this kind of enzyme. Catalase is destroyed by heating at 75 °C for 60 seconds.
What is the name of the group of substances that make up milk fat?
All fats belong to a group of chemical substances called esters, which are compounds of alcohols and acids. Milk fat is a mixture of different fatty-acid esters called triglycerides, which are composed of an alcohol called glycerol and various fatty acids. Glycerides make up almost 99 % of milk fat.
How to introduce acid to milk?
Another way of introducing an acid is through lactose-digesting bacterial cultures, which transform the lactose sugar found in milk into lactic acid, thereby lowering the pH of the milk. These bacterial cultures are used in the preparation of yogurt, certain cheeses, sour cream, and many “cultured” dairy products.
Why does milk curdle?
Acidifying milk — essentially lowering its pH — causes the milk proteins, like casein, to unwind and unfold in a process known as protein denaturing. The unfolded proteins are then free to interact with each other and clump together in a way they could not do when they were properly folded.
How is lactic acid made?
Lactic acid is produced on an industrial scale by fermentation or a synthetic method. ... The fermentation process requires carbohydrates, nutrients, and a microorganism to produce lactic acid via fermentation. The carbohydrates used in fermentation consist predominantly of hexoses or compounds which can be easily split into hexoses, e.g., glucose, corn syrups, molasses, sugar beet juice, whey, as well as rice, wheat, corn, and potato starches. ... The nutrients required by the microorganisms include soluble peptides and amino acids, phosphates and ammonium salts, and vitamins. In many cases, the peptides and amino acids are a complex nitrogen source such as yeast extract paste, corn steep liquor, corn gluten meal, malt sprouts, soy peptone, and meat peptone. Only a minimal amount of these complex nitrogen sources are used in order to simplify purification of the lactic acid. During fermentation, the pH of the broth must be controlled between 5.0 and 6.5. Lime ( calcium hydroxide ), calcium carbonate, ammonium hydroxide, and sodium hydroxide are typically used to neutralize the lactic acid made in the broth to maintain constant pH. Thus, calcium lactate, ammonium lactate, or sodium lactate salts are formed in the fermentation broth. ... Lactic acid yields are between 85 and 95% based on fermentable sugars. Typical fermentation byproducts, such as formic acid and acetic acid, are found in concentrations of less than 0.5 wt%. "Homofermentive" bacterial strains are typically used as they produce the least amount of byproducts. ... After fermentation, the lactic acid broth needs to be purified for its intended use.
What is a lactic acid?
LACTIC ACID is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in it to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas ( carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions. Slowly corrodes most metals (USCG, 1999).
How long does it take for lactic acid to reach its theoretical BOD?
AEROBIC: Lactic acid reached 22% of its theoretical BOD in 5 days using a sewage inoculum (1). In a closed bottle screening test, lactic acid, present at 2 mg/L, reached 12, 67, and 88% of its theoretical BOD after 5, 15, and 30 days, respectively, using an activated sludge inoculum (2). Lactic acid reached 59% of its theoretical BOD in 5 days using a sludge inoculum and the Warburg screening test (3). Lactic acid, present at 500 mg/L, reached 27.5, 29.4, and 33.3% of its theoretical BOD in 6, 12, and 24 hours, respectively, using an activated sludge inoculum at 2500 mg/L (4). Lactic acid was found to be easily biodegradable by biological sewage treatment (5). Lactic acid, present at 100 mg/L, reached 76% of its theoretical BOD in 2 weeks using an activated sludge inoculum at 30 mg/L in the Japanese MITI test which classified the compound as readily biodegradable (6).
How much lactic acid is excreted?
Lactic acid was found to be excreted by humans through urine at a rate of 40 mg/kg body weight/day and through sweat at 45-452 mg/100 mL (1).
How many workers are exposed to lactic acid?
According to the 2012 TSCA Inventory Update Reporting data, 7 reporting facilities estimate the number of persons reasonably likely to be exposed during the manufacturing, processing, or use of lactic acid in the United States may be as low as 10-24 workers and as high as 500-999 workers per plant; the data may be greatly underestimated due to confidential business information (CBI) or unknown values (1).
How long does lactic acid react with hydroxyl radicals?
This corresponds to an atmospheric half-life of about 2.7 days at an atmospheric concentration of 5X10+5 hydroxyl radicals per cu cm (1). The rate constant for the reaction of hydroxyl radicals in aqueous solutions at pH 1 is 4.8X10+8 L/mol-sec (2); this corresponds to an aquatic half-life of about 4.6 years at an aquatic concentration of 1X10-17 hydroxyl radicals per liter (3). Lactic acid is not expected to undergo hydrolysis in the environment due to the lack of functional groups that hydrolyze under environmental conditions (4). Laboratory studies found aqueous solutions of lactic acid to be very stable with an estimated shelf-life of 70 years (5). Lactic acid does not contain chromophores that absorb at wavelengths >290 nm and therefore is not expected to be susceptible to direct photolysis by sunlight (4).
What is the percentage of lactic acid in halibut?
The mean percentage of lactic acid in the muscle of haddock, cod, hake, flounder and halibut ranged from 0.12 to 0.26 (1).
Overview
Lactic acid is an organic acid. It has a molecular formula CH3CH(OH)COOH. It is white in the solid state and it is miscible with water. When in the dissolved state, it forms a colorless solution. Production includes both artificial synthesis as well as natural sources. Lactic acid is an alpha-hydroxy acid (AHA) due to the presence of a hydroxyl group adjacent to the carboxyl group. It is used as a synt…
History
Swedish chemist Carl Wilhelm Scheele was the first person to isolate lactic acid in 1780 from sour milk. The name reflects the lact- combining form derived from the Latin word lac, which means milk. In 1808, Jöns Jacob Berzelius discovered that lactic acid (actually L-lactate) also is produced in muscles during exertion. Its structure was established by Johannes Wislicenus in 1873.
In 1856, the role of Lactobacillus in the synthesis of lactic acid was discovered by Louis Pasteur. …
Production
Lactic acid is produced industrially by bacterial fermentation of carbohydrates, or by chemical synthesis from acetaldehyde. In 2009, lactic acid was produced predominantly (70–90%) by fermentation. Production of racemic lactic acid consisting of a 1:1 mixture of D and L stereoisomers, or of mixtures with up to 99.9% L-lactic acid, is possible by microbial fermentation. Industrial scale production of D-lactic acid by fermentation is possible, but much more challengi…
Biology
L-Lactic acid is the primary endogenous agonist of hydroxycarboxylic acid receptor 1 (HCA1), a Gi/o-coupled G protein-coupled receptor (GPCR).
During power exercises such as sprinting, when the rate of demand for energy is high, glucose is broken down and oxidized to pyruvate, and lactate is then produced from the pyruvate faster than the body can process it, causing lactate concentrations to rise. The production of lactate is bene…
Polymer precursor
Two molecules of lactic acid can be dehydrated to the lactone lactide. In the presence of catalysts lactide polymerize to either atactic or syndiotactic polylactide (PLA), which are biodegradable polyesters. PLA is an example of a plastic that is not derived from petrochemicals.
Pharmaceutical and cosmetic applications
Lactic acid is also employed in pharmaceutical technology to produce water-soluble lactates from otherwise-insoluble active ingredients. It finds further use in topical preparations and cosmetics to adjust acidity and for its disinfectant and keratolytic properties.
Lactic acid containing bacteria have shown promise in reducing Oxaluria (Kidney Stones) with its descaling properties on calcium compounds. 54
Foods
Lactic acid is found primarily in sour milk products, such as kumis, laban, yogurt, kefir, and some cottage cheeses. The casein in fermented milk is coagulated (curdled) by lactic acid. Lactic acid is also responsible for the sour flavor of sourdough bread.
In lists of nutritional information lactic acid might be included under the term "carbohydrate" (or "carbohydrate by difference") because this often includes everything other than water, protein, fa…
Forgery
Lactic acid has historically been used to assist with the erasure of inks from official papers to be modified during forgery.