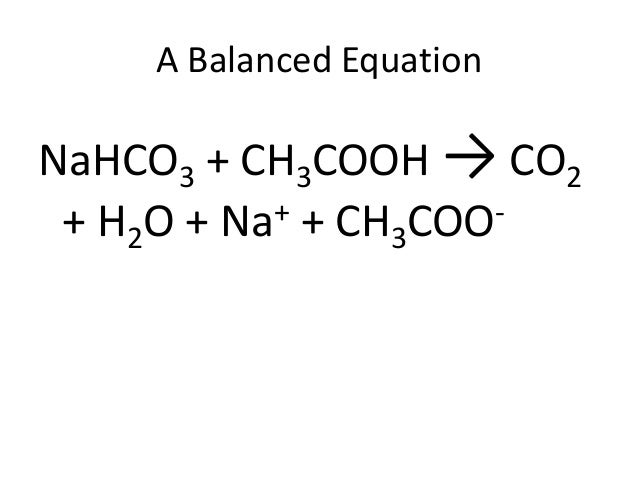
Percent composition by element
| Element | Symbol | Atomic Mass | # of Atoms | Mass Percent |
| Hydrogen | H | 1.00794 | 4 | 6.714% |
| Carbon | C | 12.0107 | 2 | 40.001% |
| Oxygen | O | 15.9994 | 2 | 53.285% |
What is the number of lone pairs in CH3COOH?
In the case of the CH3COOH molecule, two carbon atoms (C 1 and C 2) undergo hybridization. This first carbon atom is joined to the three hydrogen atoms and one right side carbon, so, the total number of attached atoms is four and it contains zero lone pairs.
What are the atoms present in CH3COOH molecule?
In the CH3COOH molecule, three types of atoms are present – hydrogen, oxygen, and carbon. It should be noted that in the lewis diagram, hydrogen atoms always go outside means they always hold the place of the surrounding position, no matter what’s the situation is.
How do you find the formal charge of CH3COOH?
⇒ Formal charge formula = (valence electrons – lone pair electrons – 1/2bonded pair electrons) All hydrogen atoms in the CH3COOH Lewis diagram have zero formal charges, just count the F.C. on carbon and oxygen atoms.
What is the Lewis structure of acetic acid CH3COOH?
CH3COOH Lewis structure contains one double bond in the functional group (COOH), 4 total lone pairs on surrounding atoms, and 8 bonded pairs attached one atom to another. Now we will draw the lewis structure of acetic acid step by step with all possible explanations.

How many atoms are present in a CH3COOH?
CH3COOH is the Acetic Acid and it is organic compoud and weak acidic in nature. it has two carbon (C) atoms, four hydrogen (H) atoms and two oxygen (O) atoms so total 8 atoms.…
What atoms are in CH3COOH?
This formula indicates that a molecule of acetic acid (Figure 2.21) contains two carbon atoms, four hydrogen atoms, and two oxygen atoms. The ratio of atoms is 2:4:2.
How many atoms are there in CH3COO?
The compound is written as Fe(CH3COO)3. In this case, there is 1 iron, 6 carbon, 9 hydrogen, and 6 oxygen atoms.
How many hydrogen atoms does CH3COOH?
4 h atomsch3cooh has 4 h atoms but only one replaceable h atom why.
How many atoms are present in a mole of CH3COOH?
As one molecule of acetic acid CH3COOH have total 8 atoms, so one mole of it contains 8×6.
How many more elements are in CH3COOH?
CH3COOH is called Acetic acid, also known as ethanoic acid and methane carboxylic acid, is a colorless liquid that has a strong and distinct pungent and sour smell. It has two carbon (C) atoms, four hydrogen (H) atoms and two oxygen (O) atoms. Because it has a carbon in its chemical formula, it is an organic compound.
How do you count atoms?
Step 1: Write the chemical formula Step 2: List all the atoms Step 3: Count the number of atoms of each element in 1 molecule. Step 4: Multiply the number of atoms of each by the coefficient. Step 5: Make sure your answer makes sense.
How many atoms are present in CaO?
Calcium oxide (CaO) molecule contains 1 calcium atom and 1 oxygen atom.
What is the number of atoms of ch3coo 2?
Percent composition by elementElementSymbol# of AtomsManganeseMn1HydrogenH6CarbonC4OxygenO4
What is the number of bonds in ch3cooh?
In acetic acid, there are seven bonds between atoms. Even though the carbonyl carbon has a double bond, there is still a -bond between the carbon and oxygen. As a result, there are seven total -bonds in a molecule of acetic acid.
How is ch3cooh formed?
Generally, this compound is produced via the reaction between methanol and carbon monoxide (carbonylation of methanol).
What are the bonds in ch3cooh?
0:372:03Is CH3COOH (Acetic acid or Ethanoic acid) Ionic or Covalent/Molecular?YouTubeStart of suggested clipEnd of suggested clipThis is a chemical bond and it's a covalent bond these electrons are shared for this right hereMoreThis is a chemical bond and it's a covalent bond these electrons are shared for this right here between the carbon and the oxygen.
How to draw lewis structure for CH3COOH (Acetic acid)
Lewis structure of CH3COOH is not much difficult to draw as you might think. We only need a good approach to draw the lewis diagram of any molecule, it doesn’t matter whether the molecule simple or complex.
Follow some steps for drawing the lewis dot structure of CH3COOH (Acetic acid)
As we know, Lewis structure or electron dot structure helps us to know, how atoms or valence electrons are arranged in a molecule. So, the first step of drawing the lewis diagram of any molecule is to determine the valence electron present within a molecule.
What is the molecular geometry of CH3COOH?
Using VSEPR theory and its chart, we can easily determine the shape or geometry of CH3COOH. “A molecular geometry is the three-dimensional arrangement of atoms within a molecule.”
Hybridization of CH3COOH
According to hybridization, “two or more orbitals overlap each other and form two or more hybrid orbitals which have same energy and shape”.
Acetic acid polarity: is CH3COOH polar or nonpolar?
Acetic acid (CH3COOH) is a polar molecule because it contains double-bonded oxygen which is more electronegative than a carbon atom, so, the difference of electronegativity in carbon and oxygen atom, generates a dipole moment in the C-O bond because of inducing a positive and negative charge on them.
Summary
The total valence electron available for drawing the Acetic acid lewis structure is 24.
How to find molar mass?
Finding molar mass starts with units of grams per mole (g/mol). When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
How to calculate molar mass?
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.