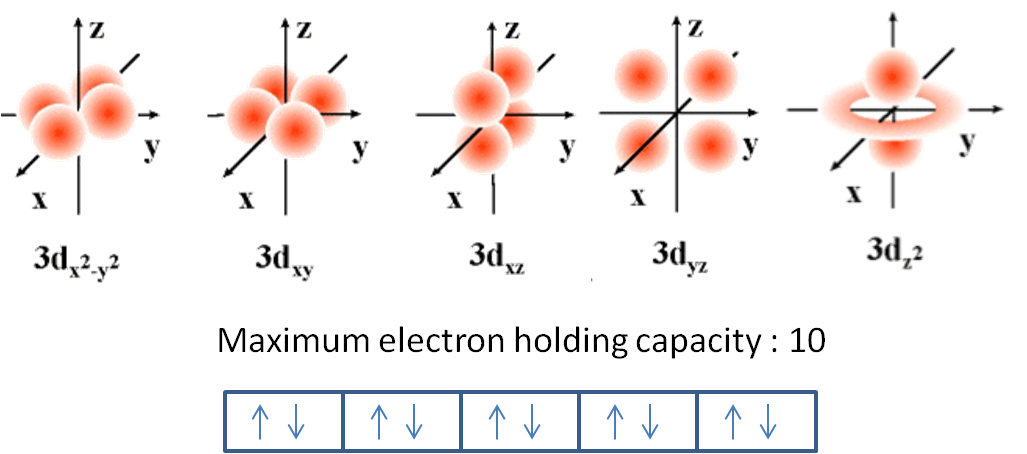
How many orbitals are there in d subshell?
There are five orbitals in D subshell. D subshell has five orbitals and each orbital can accomodate maximum 2 electrons. That's why D subshell can accomodate maximum 10 electrons. The orbitals of D subshell are as follow;
How many electrons can a d subshell accomodate?
D subshell has five orbitals and each orbital can accomodate maximum 2 electrons. That's why D subshell can accomodate maximum 10 electrons.
What is the maximum number of electrons in an orbital?
Specifically, the dz2, dx2−y2, dxy, dxz, and dyz orbitals. Furthermore, from knowing that the spin quantum number ms for an electron is ±1/2 (two spin states), and recalling the Pauli exclusion principle (two electrons in one orbital must be opposite spins), there can be a max of two electrons per orbital.
How many d orbitals are there?
5 d orbitalsThere are 5 d orbitals in the d subshell.
How many sublevels does D have?
Number of electrons per sublevelEnergy LevelSublevelsNumber of Orbitals4s1p3d5f76 more rows
What are the d orbitals?
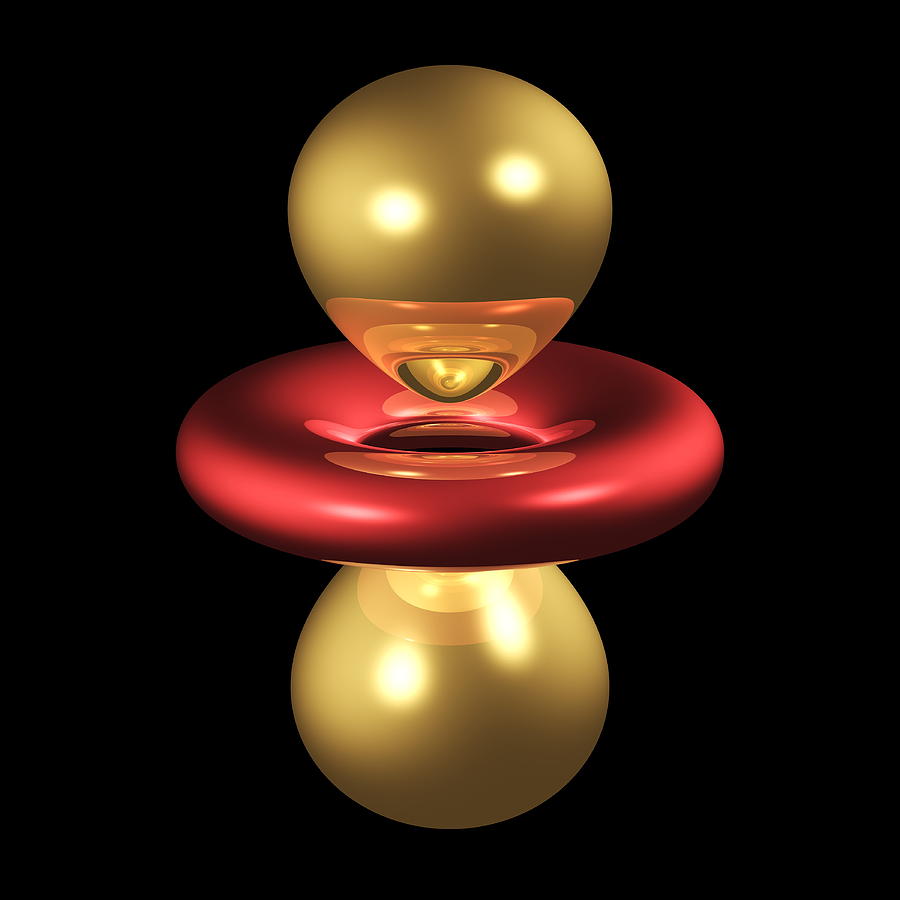
Hence, we can say that there are five d-orbitals. These orbitals are designated as dxy, dyz, dxz, dx2–y 2 and dz2. Out of these five d orbitals, the shapes of the first four d-orbitals are similar to each other, which is different from the dz2 orbital whereas the energy of all five d orbitals is the same.
Why does D have 5 orbitals?
The d subshell The angular momentum quantum number is 2, so each orbital has two angular nodes. There are 5 choices for the magnetic quantum number, which gives rise to 5 different d orbitals. Each orbital can hold two electrons (with opposite spins), giving the d orbitals a total capacity of 10 electrons.
How many orbitals are in the 5 d sublevel?
five 5d orbitalsThere are five 5d orbitals.
How many orbitals are present in the 4 d sublevel?
five 4d orbitalsThere are five 4d orbitals.