What are the First 20 Elements of the Periodic Table?
| Atomic Number | Element | Symbol |
| 1 | Hydrogen | H |
| 2 | Helium | He |
| 3 | lithium | Li |
| 4 | Beryllium | Be |
...
2.1 Electrons, Protons, Neutrons, and Atoms.
| Element | Calcium | |
|---|---|---|
| Symbol | Ca | |
| Atomic No. | 20 | |
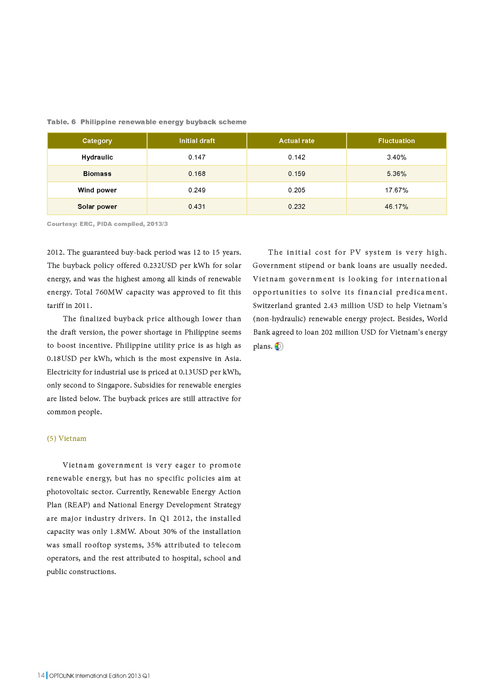
| Number of Electrons in Each Shell | First | 2 |
| Second | 8 |
What are the first 20 elements?
First 20 elements: protons, electrons and neutrons Flashcards | Quizlet Start studying First 20 elements: protons, electrons and neutrons. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
What elements have 16 protons 16 electrons and 16 neutrons?
16 protons, 16 electrons, 16 neutrons Chlorine, Cl 17 protons, 17 electrons, 18 neutrons Argon, Ar 18 protons, 18 electrons, 22 neutrons Potassium, K 19 protons, 19 electrons, 20 neutrons Calcium, Ca 20 protons, 20 electrons, 20 neutrons
How many electrons are there in an atom?
The number of electrons in an atom is some as the number of protons in the nucleus of the atom. The number of protons is called the elements atomic number. First 20 elements are as follows: Similarly berillium , boron , carbon , nitrogen , oxygen , fluorine, neon , sodium, magnesium, aluminum, silicon, phosphorus,sulphur, chlorine , argon
How many protons neutrons and electrons are there in each element?
Protons Neutrons & Electrons of All Elements (List + Images) Atomic no. Protons, Neutrons and Electrons of all t ... 24 Chromium has 24 protons, 28 neutrons and ... 25 Manganese has 25 protons, 30 neutrons an ... 26 Iron has 26 protons, 30 neutrons and 26 ... 27 Cobalt has 27 protons, 32 neutrons and 2 ... 34 more rows ...

How many electrons does element 20 have?
1 Answer. If Z , the atomic number = 20 , there MUST be 20 nuclear protons, and the element MUST be calcium, with 20 electrons associated with it.
What are the 1st 20 elements?
Lithium, Beryllium, Sodium, Magnesium, Aluminium, Potassium, and Calcium are metals in the first twenty elements. Hydrogen, Helium, Carbon, Nitrogen, Oxygen, Fluorine, Neon, Phosphorous, Sulphur, Chlorine, and Argon are the non-metals in the first twenty elements.
What are the protons for the first 20 elements?
Terms in this set (20)Hydrogen, H. 1 proton, 1 electron, 0 neutrons.Helium, He. 2 protons, 2 electrons, 2 neutrons.Lithium, Li. 3 protons, 3 electrons, 4 neutrons.Beryllium, Be. 4 protons, 4 electrons, 5 neutrons.Boron, B. 5 protons, 5 electrons, 6 neutrons.Carbon, C. 6 protons, 6 electrons, 6 neutrons.Nitrogen, N. ... Oxygen, O.More items...
What element has an electron of 20?
The element with an atomic number of 20 is Calcium Ca .
What is the Valency of 1 to 20 elements?
Valency of First 30 ElementsElementAtomic NumberValencyValency of Chlorine171Valency of Argon180Valency of Potassium (K)191Valency of Calcium20226 more rows
Who discovered the first 20 elements?
In 1869 Russian chemist Dimitri Mendeleev started the development of the periodic table, arranging chemical elements by atomic mass. He predicted the discovery of other elements, and left spaces open in his periodic table for them.
How many electrons does each element have?
Its atomic number is 14 and its atomic mass is 28. The most common isotope of uranium has 92 protons and 146 neutrons. Its atomic number is 92 and its atomic mass is 238 (92 + 146). Figure 2.2 A depiction of a helium atom....2.1 Electrons, Protons, Neutrons, and Atoms.ElementSodiumSymbolNaAtomic No.11Number of Electrons in Each ShellFirst2Second829 more columns
How do you find number of electrons?
The number of electrons in a neutral atom is equal to the number of protons. The mass number of the atom (M) is equal to the sum of the number of protons and neutrons in the nucleus. The number of neutrons is equal to the difference between the mass number of the atom (M) and the atomic number (Z).
How do you know how many electrons are in an element?
Finding the Number of Electrons The number of electrons in an atom is equal to the atomic number of an element, for neutrally charged species. This means the number of electrons and the number of protons in an element are equal. Therefore, the number of electrons in oxygen is 8.
What are the 1 to 30 elements?
Atomic Mass of First 30 ElementsATOMIC NUMBERELEMENTATOMIC MASS1Hydrogen1.0082Helium4.00263Lithium6.944Beryllium9.012226 more rows
What are the second 20 elements?
Terms in this set (20)Scandium. Sc.Titanium. Ti.Vanadium. V.Chromium. Cr.Manganese. Mn.Iron. Fe.Cobalt. Co.Nickel. Ni.More items...
What are the first 30 elements of the periodic table?
0:000:56REMEMBER FIRST THIRTY ELEMENTS OF PERIODIC TABLE ...YouTubeStart of suggested clipEnd of suggested clipToday I am going to list initial 30 elements of our modern periodic. Table.MoreToday I am going to list initial 30 elements of our modern periodic. Table.
What are the 20 elements and their uses?
ELEMENTUSES20)PotassiumFound in fertilizers21)SiliconUsed in electronics & in compounds for making glass22)SilverUsed in tableware, jewelry, photography, medicines, & coins23)SodiumSoft metal that combines with chlorine to make table salt24 more rows
How many elements are there in the periodic table?
There are 118 elements in the periodic table. Each element has a unique atomic structure that is influenced by its electronic configuration, which is the distribution of electrons across different orbitals of an atom. This article provides you with an electronic configuration chart for all these elements.
How many electrons does the M shell carry?
The M shell contains 3s, 3p, and 3d, and can carry 18 electrons. The N shell containing 4s, 4d, 4p and 4f, can carry 32 electrons. Such an arrangement helps explain the periodicity and periodic trends observed across the elements of the periodic table.
What is electronic configuration?
The concept of electronic configuration has replaced the older concept of valency and valence electrons. It involves the specific arrangement of electrons in shells and sub-shells of Bohr’s atomic model. This model has been widely accepted, and according to it, each atom has shells, which further have subshells.
What are the shells of an atom?
This model has been widely accepted, and according to it, each atom has shells, which further have subshells. The shells are labeled K, L, M, N , and so on, from the innermost to the outermost shell. Each shell has subshells that are named for the type of emission lines produced from different states of angular momentum.
What order are the first 20 elements in the periodic table?
This is also the number of protons in each atom. These are the first 20 elements, listed in order: H - Hydrogen.
How to tell how many neutrons an atom has?
This is indicated using numbers (superscripts, subscripts, or following the symbol) to give the total number of protons and neutrons.
What does the number of an element mean?
The number of the element is its atomic number, which is the number of protons in each atom of that element. The element symbol is a one- or two-letter abbreviation of the element's name. Sometimes it refers to an old name. (For instance, K is for kalium.) The element name can tell you something about its properties.
What are the names of the elements that end with gen?
Elements that have names ending with - ine belong to a group of elements called halogens. Halogens are extremely reactive and readily form compounds. Element names ending with - on are noble gases, which are inert or nonreactive gases ...
How many protons does carbon 14 have?
For example, carbon-14 has 14 protons and neutrons. Since you know all atoms of carbon have 6 protons, the number of neutrons is 14 - 6 = 8. Ions are atoms that have different numbers of protons and electrons.
What are the elements that make up a substance?
Chemical Elements. To be an element, a substance has to at least have protons, since these particles define the type of element. Elements consist of atoms , which contain a nucleus of protons and neutrons surrounded by a cloud or shell of electrons.
What does the superscript after the element symbol mean?
Ions indicated using a superscript after the element symbol that states whether the charge on the atom is positive (more protons) or negative (more electrons) and the quantity of the charge. For example, Ca 2+ is the symbol for a calcium ion that has a positive 2 charge.
What color is neon?
Neon is a colorless gas that emits a characteristic orange-red glow when excited in an electric field.
What is a group 13?
Group: group 13, p-block, considered a post-transition metal or sometimes a metalloid
Is argon a solid or a gas?
Argon is a colorless gas, liquid, and solid. It emits a bright lilac-purple glow when excited in an electric field.
Is fluorine a liquid or gas?
Fluorine is a pale yellow gas and liquid and bright yellow solid. The solid may be either opaque or translucent.
Is phosphorus a solid?
Phosphorus is a solid under ordinary conditions, but it takes several forms. The most common are white phosphorus and red phosphorus.
What is the atomic number of an element?
The atomic number of an element in the periodic table provides us with the following information: The number of electrons surrounding the nucleus in a neutral atom. In other words, we can say that the atomic number is equal to the charge on the nucleus. Hence, it is also similar to the number of protons in the nucleus.
How many protons are in the nucleus of a sodium atom?
The number of electrons is 8. Similarly, in sodium, which has an atomic number of 11, the sodium atom's nucleus consists of 11 protons. It has 11 electrons surrounding the nucleus. As we know that the atomic number is equal to the number of electrons, ...
How Does The Periodic Table Help?
The periodic table of the elements in Chemistry is the organized arrangements of all known elements in the order of their increasing atomic number represented by the number of protons of an atom.
What is the mass number of an atom?
When we add up the number of protons and the number of neutrons of the atom, it gives us the element's mass number. Neutrons and protons are collectively called nucleons because they are present in the nucleus itself. Now, let us look at a few examples. Oxygen has 8 protons, while the number of neutrons it has is also 8. This implies that the mass number of Oxygen is 8+8, that is 16.
Why are neutrons and protons collectively called nucleons?
Neutrons and protons are collectively called nucleons because they are present in the nucleus itself. Now, let us look at a few examples. Oxygen has 8 protons, while the number of neutrons it has is also 8. This implies that the mass number of Oxygen is 8+8, that is 16.
Where do protons reside?
Protons - It is a subatomic (occurring within an atom) particle. It has a positive charge. It resides in the nucleus of an atom of the element.
What is the term for elements in the same column?
Elements situated in the same column consist of similar properties and are denoted as groups. Elements located in the same row are called periods and consist of the equal highest unexcited electron energy levels. The periodic table can also provide us with information on an element's atomic weight and the usual charge. All this data, and much more, compiled into one universal, comprehensive reference table makes the periodic table important.
