Potassium-39 is composed of 19 protons, 20 neutrons, and 19 electrons. Potassium-40 is composed of 19 protons, 21 neutrons, and 19 electrons. Traces of K-40 are found in all potassium, and it is the most common radioisotope in the human body.
How many protons electrons and neutrons does potassium have?
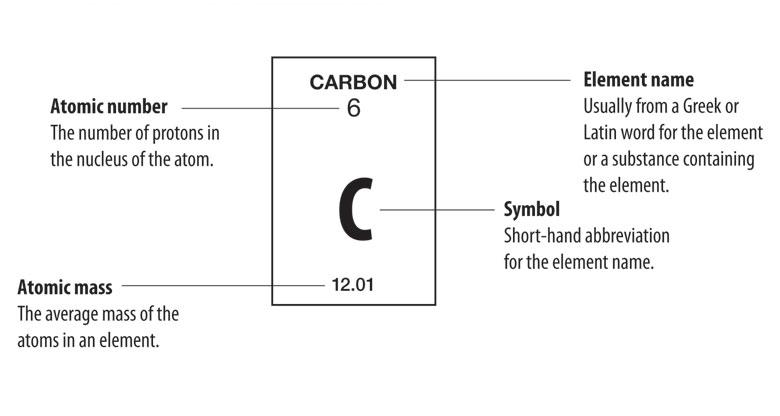
So for your question, the Periodic Table tells us that potassium has an Atomic Number of 19, so there are 19 protons and 19 electrons. The Periodic Table tells us that potassium has an Atomic Mass of ≈39. So there are 39 - 19 = 20 neutrons.
What is the difference between potassium 39 and potassium 40?
Potassium-39 is composed of 19 protons, 20 neutrons, and 19 electrons. Potassium-40 is composed of 19 protons, 21 neutrons, and 19 electrons. Traces of K-40 are found in all potassium, and it is the most common radioisotope in the human body.
What is the mass number of Typical isotopes of potassium?
Mass numbers of typical isotopes of Potassium are 39; 41. There are 25 known isotopes of potassium, three of which occur naturally: 39K (93.3%), 40K (0.0117%), and 41K (6.7%). Potassium-39 is composed of 19 protons, 20 neutrons, and 19 electrons. Potassium-40 is composed of 19 protons, 21 neutrons, and 19 electrons.
What is the number of electrons and neutrons in an atom?
The amount of electrons must be the same as the number of protons in the atom for it to be neutrally charged. So the number of electrons should also be 19. However, the number of neutrons can vary depending on the isotope. An isoto... Can we calculate how much energy is required to force a proton and an electron to combine into a neutron?

How many neutrons does potassium-39 have?
twenty neutronsArt Connections. Figure 2.1. 2: How many neutrons do (K) potassium-39 and potassium-40 have, respectively? Potassium-39 has twenty neutrons.
How many protons does potassium-39 have?
19 protonsPotassium-39 - It has 19 protons and atomic mass is 39. Hence, number of neutrons is 39 - 19 which is 20. Potassium-40 - It has 19 protons and atomic mass is 40. Hence, number of neutrons is 40 - 19 which is 21.
How many protons and neutrons are there in potassium?
19Potassium / Atomic number
How many neutrons are in potassium?
20 neutronsThe element of potassium has the symbol K. The nucleus of an atom of potassium contains 19 protons and 20 neutrons.
Where can you find the protons and neutrons?
the nucleusProtons and neutrons are heavier than electrons and reside in the nucleus at the center of the atom.
What element has 6 protons and 6 neutrons and 6 electrons?
carbonElectrons orbit in spheres around the atom. Before you start, take a look at carbon on the periodic table. It has an atomic number of 6. That means a carbon atom has 6 protons, 6 neutrons, and 6 electrons.
How many neutrons protons and electrons do potassium 39 19 K has?
Potassium-39 is composed of 19 protons, 20 neutrons, and 19 electrons.
How do u find neutrons?
For all atoms with no charge, the number of electrons is equal to the number of protons. The mass number, 40, is the sum of the protons and the neutrons. To find the number of neutrons, subtract the number of protons from the mass number. number of neutrons=40−19=21.
What element has 18 protons and 22 neutrons?
ArgonSo, the atomic number is also \[18\], and the element with atomic number \[18\] is Argon with the symbol \[Ar\]. Now we need to find the mass number of Argon. Since, a neutral atom of argon also has 22 Neutrons. So, it is clear that this is not an isotope but a neutral atom of Argon.
What element has 22 protons and 23 neutrons?
Chemical Element.com - Titanium.
How many protons and electrons are in potassium?
This tells us that in an atom of K there are 19 protons and 19 electrons.
How do you find the neutrons of potassium?
It is the number under the element symbol. For potassium it is about 39. This means that the atomic weight is 39 for both protons and neutrons. Since we know that the number of protons is 19 we can calculate the number of neutrons (39 19) as 20.
How many protons and electrons are there in potassium?
This tells us that in an atom of K there are 19 protons and 19 electrons.
Why does potassium have 20 neutrons?
It is the number under the element symbol. For potassium it is about 39. This means that the atomic weight is 39 for both protons and neutrons. Since we know that the number of protons is 19 we can calculate the number of neutrons (39 19) as 20.
How many protons neutrons and electrons does a potassium ion have?
1.17: IonsElementProtonsElectronsPotassium atom1919Potassium ion1918Sulfur atom1616Sulfur ion1618Aug 24, 2020
How many protons are in potassium 40?
19 protonsPotassium 40 contains odd numbers of both – 19 protons and 21 neutrons.
How many protons does each isotope have?
Each isotope has 19 protons and 19 electrons, but the number of neutrons vary. Potassium-39 has 20 neutrons, Potassium-40 has 21 neutrons and Potassium-41 has 22 neutrons. Therefore, each isotope of an element will have a different mass number but the same atomic number.
How many neutrons are in carbon?
The number of neutrons is equal to the Atomic Mass minus the Atomic Number. So for your question, the Periodic Table tells us that carbon has an Atomic Number of 6, so there are 6 protons and 6 electrons. The Periodic Table tells us that carbon has an Atomic Mass of ≈12. So there are 12 - 6 = 6 neutrons.
What happens when electrons leave a metal atom?
For example, a chlorine atom that is hungry for an electron (to fill its outer shell) will gladly accept one from potassium in the following reaction: K + Cl → K+ + Cl- (or just KCl, an ionic compound composed of K+ and Cl-). Another possibility is that the K encounters K+ and just hands its electron over! This is a little like what happens when a metal conducts electricity — the electron easily jumps from one metal atom to its neighbor and this happens lots of times, very fast.
Why is the mass of an element equal to the sum of its protons and neutrons?
The mass of an element is equal to the sum of its protons and neutrons because each one weighs roughly 1 atomic mass unit (amu).
How to find the atomic mass of neutrons?
You can usually get the Atomic Mass from the same Table of the Elements. The number of neutrons is equal to the Atomic Mass minus the Atomic Number.
How many electrons are needed to neutralize an atom?
The amount of electrons must be the same as the number of protons in the atom for it to be neutrally charged. So the number of electrons should also be 19.
What is the number in the top left of an element?
Let's first talk about the numbers associated with the element and what they mean. The number in the top left is called the atomic number. Not only does it show where it would be located on the periodic table, it tells us how many protons are in the nucleus of a single potassium atom.