
Can an insulator have more than 4 electrons?
Even insulators have more than 4 electrons in its valence shell, so why are they not conducting electricity? - Quora Even insulators have more than 4 electrons in its valence shell, so why are they not conducting electricity?
Why are valence electrons in an insulator below the conduction band?
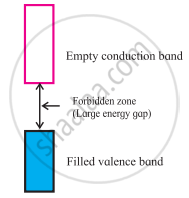
In an insulator, the valence electrons are all in Fermi energy levels that are below the conduction band for that material, and it is an insulator. Applying a voltage to an insulator will not "lift" the valence electrons up into the conduction band to allow them to support current flow.
How many valence electrons does a semiconductor have?
Remember that a good conductor has 1 valence electrons and an insulator has eight valence electrons. The semiconductor has 4 valence electrons. It is neither a good conductor or a good insulator. When the number of protons in an atom equals the number of electrons the atom is said to be neutral.
How many electrons does an atom have in its valence shell?
One atom has two electrons in its valence shell and another has seven. Which is the insulator and which is the conductor? The atom is neither insulator nor conductor. the material made from said atoms might be one or the other (or neither).
Do insulators have electrons?
Insulators are materials whose atoms have tightly bound electrons. These electrons are not free to roam around and be shared by neighboring atoms. Some common insulator materials are glass, plastic, rubber, air, and wood.
Do insulators have free valence electrons?
Materials with high electron mobility (many free electrons) are called conductors, while materials with low electron mobility (few or no free electrons) are called insulators.
Do conductors have valence electrons?
Most commonly used electrical conducting material is aluminium and it has three valence electrons, and another metallic conductor is magnesium which has two valence electrons. The most popularly known electrical conductor is copper, and the copper atom has only one valence electron.
How many valence electrons do conductors semiconductors and insulators have?
Remember that a good conductor has 1 valence electrons and an insulator has eight valence electrons. The semiconductor has 4 valence electrons. It is neither a good conductor or a good insulator. When the number of protons in an atom equals the number of electrons the atom is said to be neutral.
How many free electrons does an insulator have?
An atom hav- ing a relatively small number of electrons in its outer shell in comparison to the number of elec- trons required to fill the shell will easily lose these valence electrons. valence electrons, an insulator has five or more valence electrons, and semiconductors usually have four valence electrons.
How are conductors different from insulators?
Conductors allow for charge transfer through the free movement of electrons. In contrast to conductors, insulators are materials that impede the free flow of electrons from atom to atom and molecule to molecule.
What is conductor or insulator?
Materials that do not permit heat and electricity to pass through it. A few examples of a conductor are silver, aluminum, and iron. A few examples of an insulator are paper, wood, and rubber. Electrons move freely within the conductor. Electrons do not move freely within the insulator.
What are the characteristics of an insulator?
Important Properties of InsulatorsProperty 1: In an insulator, the valence electrons are tightly held together. ... Property 2: The ability of the material to not allow the electric current to pass through it is called electrical resistance. ... Property 3: Insulators have large dielectric strength.More items...
What is the valence electron of Li?
The valence electron on Li is a 2s electron (in its ground state). That 2s electron will feel the pull of 3 protons and the repulsion of 2 inner (1s) electrons. The “core” is the nucleus and the inner electrons. Hence the “core Charge” can be approximated as the number of protons minus the number of inner electrons.
How many electrons are in an octet?
Scientists consider that as due to of having 8 electrons in their outermost shell, and the ionization energy of these atoms are very high and their electron affinties are very low. An octet's rule had been invented as “in chemical reactions, atoms share, accept or gain electron to achieve eight ele. Continue Reading.
What are the outermost electrons in an atom?
Valence electrons in an atom are the outermost electrons, the least bound to the central positive charge. In a solid substance, there is often a crystalline structure of the atoms or molecules, the atoms or molecules form a lattice in space.
What happens if there are no electrons in the conduction band?
If there are no electrons in the conduction band, the material cannot conduct electricity. In conductors — like metals — the conduction band actually overlaps with the valence band, which means that electrons can easily move from one to the other.
What is the outermost energy level of an atom?
The outermost energy level of an atom is known as the valence energy level , and the electrons in the valence energy level are called valence electrons. A sodium atom has one valence electron. When two atoms interact, things get a bit more complicated.
What is the net core charge of Li?
The net core charge is the charge of the protons minus the sum of the charges on all the inner electrons. Li has a atomic number Z=3 meaning three protons in the nucleus and 3 electrons in the neutral atom. The valence electron on Li is a 2s electron (in its ground state).
What happens when you apply a small potential difference across a conductor?
The application of even a small potential difference across a conductor will cause electrons to move more-or-less freely, with only minimal resistance. In a semi-conductor — like silicon — the conduction band is just slightly above the valence band in terms of energy.
How many electrons are in a cubic centimeter of copper?
and electronic industries. A cubic centimeter of copper (about the size . of a thimble) contains approximately 8.4 × 1022free electrons at room . temperature. There are many factors, which influence the choice of conductor in a .
How many protons does copper have?
contains 29 protons. A neutral copper atom must therefore have 29 . electrons distributed amongst its various shells. Shells k, 1, and mare . filled to capacity with a total of 28 electrons, so there is only one . electron in the nshell. The outermost shell of an atom, the nshell in . this case, is called the valence shell, ...
What is it called when an electron breaks away from its parent?
When an . electron breaks away from its ‘parent’ atom, it is called afree . electron, since it is then free to wander randomly through the . material. An atom producing such a free electron acquires a net positive . charge, because its total number of protons is then one greater than . its total number of electrons.
What is the transfer of charge in conductors?
actually chargedparticles that do the moving. In conductors, the . transfer of charge occurs only as a result of the motion of electrons, . and for that reason, electrons are often called charge carriers.
What are the materials that hold electrons tightly?
In some materials such as glass, rubber, wood and most plastics, the electrons are held quite tightly and are not free to move easily from place to place. These materials are called insulators. In other materials such as copper. 5. , silver.
What is the flow of electrons in a battery?
The flow of electrons is known as an electrical current. An electrical current flows when you connect one end of a battery. 19. to the other with a conductor, with the electrons flowing from the negative side of the battery to the positive side much like water flows in a pipe.
Why does rubbing on a slide cause electrons to be moved from the child to the slide?
This leaves the child positively charged. Because people can conduct electricity, the electrons came from all parts of his body, including his hair . We see the evidence.
What is the atom made of?
Understanding atoms is key to understanding how electricity works. Atoms are made of protons, neutrons and electrons. An atom#N#2#N#consists of one or more positively charged protons bound to one or more neutral neutrons. These are found in the centre of an atom called the nucleus#N#3#N#, with one or more negatively charged electrons moving around them.
Overview
Physics of conduction in solids
Electrical insulation is the absence of electrical conduction. Electronic band theory (a branch of physics) dictates that a charge flows if states are available into which electrons can be excited. This allows electrons to gain energy and thereby move through a conductor such as a metal. If no such states are available, the material is an insulator.
Most (though not all, see Mott insulator) insulators have a large band gap. This occurs because t…
Uses
A very flexible coating of an insulator is often applied to electric wire and cable; this assembly is called insulated wire. Wires sometimes don't use an insulating coating, just air, since a solid (e.g. plastic) coating may be impractical. However, wires that touch each other produce cross connections, short circuits, and fire hazards. In coaxial cable the center conductor must be supported precisely in the middle of the hollow shield to prevent EM wave reflections. Finally, wir…
Insulation in electrical apparatus
The most important insulation material is air. A variety of solid, liquid, and gaseous insulators are also used in electrical apparatus. In smaller transformers, generators, and electric motors, insulation on the wire coils consists of up to four thin layers of polymer varnish film. Film-insulated magnet wire permits a manufacturer to obtain the maximum number of turns within the available spa…
Telegraph and power transmission insulators
Overhead conductors for high-voltage electric power transmission are bare, and are insulated by the surrounding air. Conductors for lower voltages in distribution may have some insulation but are often bare as well. Insulating supports called insulators are required at the points where they are supported by utility poles or transmission towers. Insulators are also required where the wi…
Insulation of antennas
Often a broadcasting radio antenna is built as a mast radiator, which means that the entire mast structure is energised with high voltage and must be insulated from the ground. Steatite mountings are used. They have to withstand not only the voltage of the mast radiator to ground, which can reach values up to 400 kV at some antennas, but also the weight of the mast construction and dynamic f…
See also
• Stephen Gray – English physicist
• Dielectric material
• Electrical conductivity