How often is the Food Code updated?
Between 1993 and 2001, the Food Code was issued in its current format, every two years. With the support of the Conference for Food Protection (CFP), FDA decided to move to a four-year interval between complete Food Code editions.
What is the FDA Food Code?
FDA Food Code. It represents FDA’s best advice for a uniform system of provisions that address the safety and protection of food offered at retail and in food service. This resource is recommended as a public health guide and for community interventions. This model is offered for adoption by local, state, and federal governmental jurisdictions...
What are the 2017 Food Code recommendations?
2017 Recommendations of the United States Public Health Service Food and Drug Administration The Food Code is a model for safeguarding public health and ensuring food is unadulterated and honestly presented when offered to the consumer. It represents FDA's best advice for a uniform system of provisions that address the safety and
What is the Food Code Revision and publication cycle?
THE CODE REVISION PROCESS (A) Food Code Revision and Publication Cycles FDA is issuing a new edition of the Food Code every 4 years. During the 4-year span of time between editions, FDA may issue supplements to an existing edition. Each new edition will incorporate the changes made in the supplement as well as any new revisions.

What is the current FDA Food Code?
The Food Code is a model for safeguarding public health and ensuring food is unadulterated and honestly presented when offered to the consumer. It represents FDA's best advice for a uniform system of provisions that address the safety and protection of food offered at retail and in food service.
WHO updates and writes the Food Code?
The FDAThe FDA writes the food code with input from regulatory officials (USDA and CDC), industry, academia, and consumers at an industry meeting of the Conference for Food Protection (CFP). The Food Code used to be updated and published every two years, the process to develop a Food Code revision is really time consuming.
Is the Food Code FDA federal law?
The FDA Food Code is not federal law. It is the FDA's best “advice” for ways to ensure that food at retail and in foodservice is safe, properly protected and presented. It is up the agencies that have responsibility for food safety to either adopt or adapt the FDA code to their own jurisdiction.
How many states have adopted the FDA Food Code?
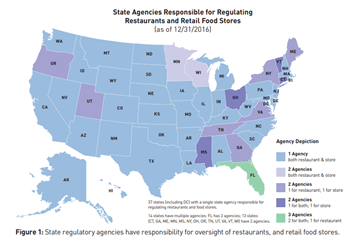
Of the 64 State regulatory agencies, 62 State agencies have adopted codes patterned after the 2017, 2013, 2009, 2005, 2001, or 1995 versions of the FDA Food Code, whereas 2 State agencies have not adopted the FDA Food Code (Table 2).
What year was the most recent FDA Food code updated?
2017The most recent Food Code, the 9th edition, was released in 2017.
Who writes the codes that regulate retail and food service operations?
Safe Serv chapter 1QuestionAnswerWhich government agency writes the codes that regulate retail and foodservice operations?State and local health departmentsWhich government agency conducts research into the causes of foodborne-illness outbreaks?CDC and PHSWhich government agency inspects meat, poultry and eggs?USDA14 more rows
Which statement about the FDA Food code is true?
Which is true about the FDA Food Code? It provides recommendations for food safety regulations. How long does a foodservice operation typically have to correct a violation of a priority item? What determines how often a foodservice operation is inspected?
How does FDA regulate food?
The FDA approves food additives in food for people. Although the FDA does not have premarket approval of food products, it has the authority to approve certain ingredients before they are used in food or intended to contact food.
When was the first Food Code published?
The Food Code originated from the Pure Drug and Food Act of 1906. This law was instituted, because food manufactures were producing and selling impure products for consumption.
Is the Food Code FDA a federal law 360 training?
Food code is neither a federal law or regulation, rather it is the FDA best advice for uniform inspection or regulation of food safety. Each state, county, and city health department has the option to adopt all or part of the code, thus food safety regulations often vary in different health department jurisdictions.
Do Different states have different food regulations?
There appear to be no major fundamental differences between state and federal food safety laws, although some state laws are based on the 1906 Pure Food and Drugs Act and others on the 1938 Federal Food, Drug, and Cosmetic Act (FDA, 1993).
Does FDA regulate grocery stores?
Examples of Food businesses NOT regulated by FDA: Retail food establishments (i.e. grocery stores, restaurants, cafeterias, and food trucks), which are regulated by state and local governments. Farmers markets.
What is the Food Code 2017?
Food Code 2017. The Food Code is a model for safeguarding public health and ensuring food is unadulterated and honestly presented when offered to the consumer. It represents FDA's best advice for a uniform system of provisions that address the safety and protection of food offered at retail and in food service.
What are the benefits of the 2017 Food Code?
The benefits associated with complete and widespread adoption of the 2017 Food Code as statutes, codes and ordinances include: Reduction of the risk of foodborne illnesses within food establishments, thus protecting consumers and industry from potentially devastating health consequences and financial losses.
What is uniform standards?
Uniform standards for retail food safety that reduce complexity and better ensure compliance. The elimination of redundant processes for establishing food safety criteria. The establishment of a more standardized approach to inspections and audits of food establishments.
General
Numerous editing changes were made throughout the document for internal consistency, to correct some errors in the 2013 Code and for clarification.
Preface
Amended Preface sections 5 and 7 to add updated information and revise dates to make current.
Chapter 2 Management and Personnel
Amended the Food Code and its Annexes, where applicable, to revise the descriptors of illness caused by Salmonella Typhi or nontyphoidal Salmonella. This change allows the use of plain language descriptors to simplify the restriction and exclusion criteria.
Chapter 3 Food
Added new subparagraph 3-302.11 (A) (1) (c) to indicate separating raw animal foods during storage, preparation, holding and display from fruits and vegetables before they are washed and re-designated existing subparagraph 3-302.11 (A) (1) (c) as new subparagraph 3-302.11 (A) (1) (d).
Chapter 7 Poisonous or Toxic Materials
Amended paragraph 7-204.12 (A) to re-designate it as the lead in paragraph for this section.
Annex 2 References
Amended to update web link and date for reference (#11- Pathogens Transmitted by Food Contaminated by Infected Persons Who Handle Food, and Modes of Transmission of Such Pathogens).
Annex 4 Management of Food Practices-Achieving Active Managerial Control of Foodborne Illness Risk Factors
Amended Table 2a Naturally Occurring Chemical Hazards at Retail, Along with Their Associated Foods and Control Measures to correct the spelling of the term “Tetrodoxin” to “Tetrodotoxin” located in the third row.
What is the Food Code?
The Food Code is a model for safeguarding public health and ensuring food is unadulterated and honestly presented when offered to the consumer. It represents FDA's best advice for a uniform system of provisions that address the safety and protection of food offered at retail and in food service. This model is offered for adoption by local, state, ...
What is the FDA guidance?
This guidance represents FDA's current thinking on safeguarding public health and ensuring food is unadulterated and honestly presented when offered to the consumer. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public.
FDA Food Code
The Food Code is guidance representing FDA’s current thinking and is a model on safeguarding public health and ensuring food is unadulterated and honestly presented when offered to the consumer. It represents FDA’s best advice for a uniform system of provisions that address the safety and protection of food offered at retail and in food service.
Evidence-Based Resource Details
U.S. Department of Health and Human Services, U.S. Food and Drug Administration [Internet]. FDA Food Code. c2013– [cited 2014 Mar 12]. Available from: www.fda.gov/FoodCode

General
- Numerous editing changes were made throughout the document for internal consistency, to correct some errors in the 2013 Code and for clarification.
- Updated the web links throughout the Code and Annexes.
- Converted several Tables, charts, and images throughout the Code to meet web accessibility requirements under Section 508 of the Rehabilitation Act of 1973 (29 U.S.C. 794d). Section 5…
- Numerous editing changes were made throughout the document for internal consistency, to correct some errors in the 2013 Code and for clarification.
- Updated the web links throughout the Code and Annexes.
- Converted several Tables, charts, and images throughout the Code to meet web accessibility requirements under Section 508 of the Rehabilitation Act of 1973 (29 U.S.C. 794d). Section 508 mandates th...
Chapter 1 Purpose and Definitions
- Added new term “Intact Meat” Revised “Vending Machine” to be more inclusive of the diverse means of payment available.
Chapter 2 Management and Personnel
- Amended the Food Code and its Annexes, where applicable, to revise the descriptors of illness caused by Salmonella Typhi or nontyphoidal Salmonella. This change allows the use of plain language descriptors to simplify the restriction and exclusion criteria. 2-102.12 Amended paragraph 2-102.12(A) to state that the Person in Charge shall be the Certified Food Protection …
Chapter 3 Food
- 3-302.11 Added new subparagraph 3-302.11(A)(1)(c) to indicate separating raw animal foods during storage, preparation, holding and display from fruits and vegetables before they are washed and re-designated existing subparagraph 3-302.11(A)(1)(c) as new subparagraph 3-302.11(A)(1)(d). 3-401.11 Amended subparagraph 3-401.11(A)(1)(b) to include the term intact …
Chapter 4 Equipment, Utensils, and Linens
- Part 4-3 Numbers and Capacities Amended to add new Subpart 4-303, Cleaning Agents and Sanitizers. Amended to add new Section 4-303.11 Cleaning Agents and Sanitizers, Availability, to require that equipment and utensil cleaning agents and sanitizers be provided and readily accessible for use.
Chapter 7 Poisonous Or Toxic Materials
- 7-204.12 Amended paragraph 7-204.12(A) to re-designate it as the lead in paragraph for this section. Amended subparagraphs 7-204.12(A)(1)-(4) by re-designating them as paragraphs 7-204.12(A)-(D) in order to be inclusive of all washing chemicals and antimicrobial agents that may be used in the washing and treatment of produce as specified in 21 CFR 173. Deleted existing p…
Chapter 8 Compliance and Enforcement
- 8-103.12 Amended paragraph 8-103.12(B) to replace existing cross reference to paragraphs 8-201.14 (D) and (E) with a cross reference to paragraph 8.201.14 (D) and subparagraph (E)(3). 8-201.14 Amended to: 1. add new paragraph (A) to add a new specification for the permit holder or permit applicant to include general information with the HACCP plan submission; 2. add a new p…
Annex 2 References
- 2-102.12 Amended to update web link and date for reference (#11- Pathogens Transmitted by Food Contaminated by Infected Persons Who Handle Food, and Modes of Transmission of Such Pathogens). 3. SUPPORTING DOCUMENTS Amended to add new section, U. Sanitation Practices Standard Operating Procedures and Good Retail Practices to Minimize Contamination and Grow…
Annex 3 Public Health Reasons/Administrative Guidelines
- 2-103.11 Amended Public Health Reasons for §2-103.11 Person in Charge (Duties) to add a new paragraph 5 that describes why paragraphs (G), (H) and the new (I) are important to the safe operation of a food establishment and to update the paragraphs referencing (M) and (N) to reflect the updated designations for paragraphs that follow the new (I). 2-201.11 Amended Public Healt…
Annex 6 Food Processing Criteria
- Amended Section 2. Reduced Oxygen Packaging, Section (I), Hazard Analysis and Critical Control Point (HACCP) Operation to revise the introductory paragraph to replace the existing cross reference to paragraph 8-201.14(D) with a cross reference to Section 8-201.14.