The plum pudding model (also known as Thomson
Joseph John Thomson
Sir Joseph John Thomson OM PRS was an English physicist and Nobel Laureate in Physics, credited with the discovery and identification of the electron, the first subatomic particle to be discovered.
What was wrong with the 'plum pudding' model?
Why is the Plum Pudding Model Wrong? In 1911, Rutherford proved that the Thomson hypothesis was "wrong": there was no uniform distribution of both positive and negative particles. Rutherford has shown the atom has a small, massive, positively charged nucleus in it.
What did the pudding represent in the plum pudding model?
Thomson’s Plum Pudding Model is the first model to represent the atomic structure of matter. According to Thomson’s Plum Pudding Model, a substance is consists of small spheres which are having the radius of about 10 -10 m in diameter. The positive charge is spread uniformly throughout the volume of sphere called pudding.
What does the plum pudding model represent?
What does the plum pudding model represent? The plum pudding model depicts the electrons as negatively-charged particles embedded in a sea of positive charge. The structure of Thomson’s atom is analogous to plum pudding, an English dessert (left).
What did the plum pudding model prove?
What did the plum pudding model prove? Thomson’s experiments with cathode ray tubes showed that all atoms contain tiny negatively charged subatomic particles or electrons. Thomson’s plum pudding model of the atom had negatively-charged electrons embedded within a positively-charged “soup.”
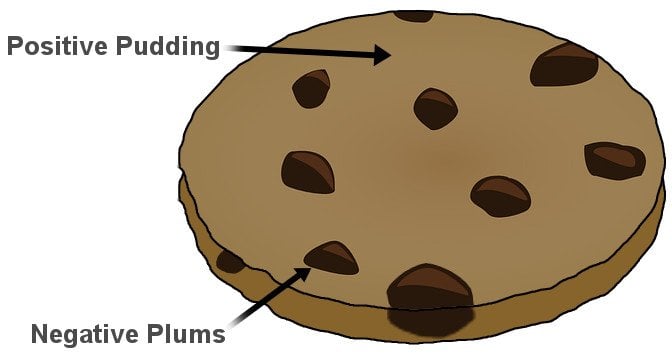
What was the plum pudding model and how did it describe the atom?
Thomson's plum pudding model of the atom had negatively-charged electrons embedded within a positively-charged "soup." Rutherford's gold foil experiment showed that the atom is mostly empty space with a tiny, dense, positively-charged nucleus. Based on these results, Rutherford proposed the nuclear model of the atom.
Why was it called plum pudding model?
These corpuscles would later be named “electrons”, based on the theoretical particle predicted by Anglo-Irish physicist George Johnstone Stoney in 1874. And from this, the Plum Pudding Model was born, so named because it closely resembled the English desert that consists of plum cake and raisins.
Which best describes the plum pudding model of JJ Thomson?
The Plum-pudding model is a model of the atom proposed by J.J Thompson in 1904. According to the Plum-Pudding model, an atom is composed of negatively charged electrons distributed in a uniformly positively charged volume of the atom just like plums are distributed in a pudding.
What did the plum pudding model prove?
At the time of discovery, J.J. Thomson called this negatively charged particle a corpuscles. Thomson's Plum Pudding Model is the first model to represent the atomic structure of matter. The positively charged sphere exerts the force on negatively charged electrons.
Which of the following is the best description of the plum pudding model of an atom?
Option C represents the plum pudding model given by Thomson. He proposed that electrons are embedded in the positively charged sphere just like plums embedded in the pudding.
What is the plum pudding model of an atom quizlet?
He also discovered that these particles had mass. As a result of this work he proposed the plum pudding model of the atom in which negatively charged electrons were scattered throughout a lump of positive charge like raisins in plum pudding. Thomson also measured the charge to mass ratio of electrons.
What was JJ Thomson experiment called?
experiment Cathode Ray Tube (CRT)The experiment Cathode Ray Tube (CRT) conducted by J. J. Thomson, is one of the most well-known physical experiments that led to electron discovery. In addition, the experiment could describe characteristic properties, in essence, its affinity to positive charge, and its charge to mass ratio.
What did JJ Thomson call his model?
In 1897, J. J. Thomson discovered the first subatomic particle, the electron, while researching cathode rays. To explain the neutrality of atoms, Thomson proposed a model of the atom in which negative electrons are scattered throughout a sphere of positive charge. He called his atom the plum pudding model.
Which best describes the current atomic model?
Which best describes the current atomic theory ? Atoms are composed of electrons in different clouds around a positive nucleus. 8.
1. What was JJ Thomson's Atomic Model?
J J.J. Experiments with cathode ray tubes by Thomson showed that all the atoms contain tiny subatomic particles or electrons that are negatively ch...
2. Why was JJ Thomson's Model Wrong?
At the time, Thomson's model was correct, because it explained everything scientists already understood about the atom. Thomson 's model was dismis...
3. Explain JJ Thomson's Contribution to the Atomic Theory?
In the year 1897 J.J. Thomson discovered the electron by playing with a tube that was Crookes, or cathode ray. He had shown that the cathode rays w...
4. Why is the Plum Pudding Model Wrong?
In 1911, Rutherford proved that the Thomson hypothesis was "wrong": there was no uniform distribution of both positive and negative particles. Ruth...
5. What is the Importance of JJ Thomson’s Atomic Model?
JJ Thomson’s discovery in 1897 was a revolution for its time and a landmark occasion in the history of particle physics. In this model, for the fir...
6. Why is Thomson’s Atomic model also known as the Watermelon Model?
The JJ Thomson model is also called the atomic watermelon model because it resembles both spherical plum pudding and watermelon. It is also compare...
7. What was the Rutherford Experiment?
In 1909, the physicist Rutherford along with Ernest Marsden performed an experiment which is known as the Rutherford alpha scattering experiment wa...
8. What is an Alpha Particle?
An Alpha particle, also known as alpha rays or alpha radiation, consists of protons and neutrons bound together into a particle which is identical...
9. What do the Latest study on Electrons and the Model of the Atom tell us?
According to the latest research, The orbital theory of elections has been the most exciting field where electrons are considered as clouds of nega...
What was the significance of the Plum Pudding Model?
His work in determining that atom’s were divisible, as well as the existence of electromagnetic forces within the atom, would also prove to be major influence on the field of quantum physics.
Why is the plum pudding model named after the plum pudding?
And from this, the Plum Pudding Model was born, so named because it closely resembled the English desert that consists of plum cake and raisins.
Why is the Plum Pudding Model important?
Though defunct by modern standards, the Plum Pudding Model represents an important step in the development of atomic theory. Not only did it incorporate new discoveries, such as the existence of the electron, it also introduced the notion of the atom as a non-inert, divisible mass. Henceforth, scientists would understand that atoms were themselves composed of smaller units of matter and that all atoms interacted with each other through many different forces.
Which model of the atom is surrounded by an equal number of electrons in orbital shells?
the Bohr Model ).
When was the atomic model first proposed?
Ever since it was first proposed by Democritus in the 5th century BCE, the atomic model has gone through several refinements over the past few thousand years. From its humble beginnings as an inert, indivisible solid that interacts mechanically with other atoms, ongoing research and improved methods have led scientists to conclude ...
What was the smallest atom in the 19th century?
However, most scientists ventured that this unit would be the size of the smallest known atom – hydrogen. By the end of the 19th century, the situation would change drastically.
Who proposed the plum pudding atomic model?
The plum pudding atomic model or atomic theory is one of the earlier atomic theories. The model was proposed by J. J. Thomson, who is also known for the discovery of the electron. From his cathode-ray tube experiments, he realized that atoms consisted of negatively particles (electrons), which he called corpuscles. He imagined an atom as negatively charged particles floating in the positively charged soup and put forward his theory in 1904.
What is the name of the model of the cloud of uniform positive charge?
Hence, the model got its name the plum pudding model. Comparison between plum pudding and atom.
What did Thomson conclude about the size of particles?
Using this ratio, Thomson concluded the size of these particles was even smaller than of a hydrogen ion. At that time, it was known the atoms were electrically neutral. To explain this, he introduced the presence of the positive charge in an atom such that the positive charge counterbalances the negative charge.
Why was Rutherford's gold scattering experiment rejected?
The model was rejected because it was ineffective to justify light spectrum.
What is the shape of an atom?
According to Thomson, the atom was spherical in shape. The atom consisted of the negatively charged particles. The negatively charged particles or electrons were floating in a positively charged soup (or ocean). An atom was electrically neutral. The positive and negative charge in the atoms were equal.
What is the watermelon model of atom?
This model is also called as watermelon model of atom because it resembles a spherical plum pudding as well as a watermelon.
What is the red part of a watermelon compared to?
The model was also compared with a watermelon because the red edible part of a watermelon was compared to the positively charged sphere and the black seeds filling the watermelon looked similar to the electrons inside the sphere. An atom consists of a positively charged sphere, and it embeds the electrons.
What was the goal of the atomic model?
The goal of each atomic model was to present all the experimental evidence of atoms in the simplest way possible. Thomson proposed the atom should be composed of electrons surrounded by a broth of positive charge to counteract the negative charges of the electrons. Which led to plum pudding model.
Which experiment showed that all atoms contain tiny subatomic particles or electrons that are negatively charged?
J J.J. Experiments with cathode ray tubes by Thomson showed that all the atoms contain tiny subatomic particles or electrons that are negatively charged. Thomson suggested the atom's plum pudding model, which had negatively charged electrons trapped in a "soup" filled with positive effect.
Why was Thomson's model correct?
At the time, Thomson's model was correct, because it explained everything scientists already understood about the atom. Thomson 's model was dismissed by the Japanese physicist Hantaro Nagaoka. He said a massive nucleus was in the atom. The electrons, like the rings revolving around Saturn, revolved around the nucleus.
Who invented the electron model?
Plum Pudding Model. J. J. Thomson, who invented the electron in the year 1897, suggested the atom's plum pudding model in 1904 before the atomic nucleus was found, to include the electron in the atomic model. In this model, the atom consists of electrons (which Thomson called them "corpuscles") surrounded by a soup of positive charge ...
Which model fails to explain Rutherford's -particle scattering experiment?
Thomson's atom model fails to explain Rutherford's α-particle scattering experiment in which most of the fast moving α - particles went through the gold foil straight away.
Which model suggested that atoms are composed of positive and negatively charged particles?
J. J. Thomson's 'plum-pudding' model suggested that atoms are composed of positive and negatively charged particles. The electrons that are negatively charged are embedded (like the plums in a pudding) in a sea of positive charge.
Which model suggested that electrons in an atom are embedded in a positively charged substrate?
While the plum pudding model suggested that electrons in an atom are embedded in a positively charged substrate, Rutherford's experiment concluded that it is only the core of an atom that is positively charged around which the electrons move in fixed orbits.
What was Thomson's atom?
Thomson saw the atom to be a spherical cloud of positive proton matter with electrons dispersed throughout it . Then the gold foil experiment is when Rutherford shot alpha particles at a sheet of gold foil and when there was deflection he concluded there was a dense part in the center of an atom with he called the nucleus made up of neutrons and protons.
Who proposed the Thomson model?
Thomson atomic model was proposed by William Thomson in the year 1900. This model explained the description of an inner structure of the atom theoretically. It was strongly supported by Sir Joseph Thomson, who had discovered the electron earlier.
Why did Thomson's atomic model fail to explain the stability of an atom?
It failed to explain the stability of an atom because his model of atom failed to explain how a positive charge holds the negatively charged electrons in an atom. Therefore, This theory also failed to account for the position of the nucleus in an atom.
What was the negative particle in the Cathode Ray Tube experiment?
Thomson. This experiment took place in the year 1897. Cathode ray tube is a vacuum tube. The negative particle was called an electron.
Did Thomson's model explain the scattering of alpha particles?
Thomson’s model failed to explain the scattering of alpha particles by thin metal foils . No experimental evidence in its support. Although Thomson’s model was not an accurate model to account for the atomic structure, it proved to be the base for the development of other atomic models. Find the atomic structure pdf here.
